Ema Product Information Templates
Ema Product Information Templates - Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Web changes will enhance presentation of information for patients and healthcare professionals. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. The committee for medicinal products for human use ( chmp) and committee on advanced. The template for each european language, as well as an annotated template in english, are available on both the. Web the ema just released a new product information template for public consultation. Changing the labelling and package leaflet (article 61(3) notifications). Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Web electronic product information (epi) for human medicines across the european union1. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Changing the labelling and package leaflet (article 61(3) notifications). Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. The template for each european language, as well as an annotated template in english, are available on both. Web documents providing officially approved information for healthcare professionals and patients on a medicine. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web the european medicines agency will review new information on this. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. The committee for medicinal products for human use ( chmp) and committee on advanced. Best practice guide for the processing of spc, labelling and package leaflet and the. Web this template is used by companies to create the product information. Changing the labelling and package leaflet (article 61(3) notifications). Best practice guide for the processing of spc, labelling and package leaflet and the. Web processing of spc, labelling and packaging. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web the update introduces certain modifications to the human. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web documents providing officially approved information for healthcare professionals and patients on a medicine. Web this template is. Web the ema just released a new product information template for public consultation. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Changing the labelling and package leaflet (article 61(3) notifications). Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web changing the (invented) name. Web changes will enhance presentation of information for patients and healthcare professionals. Web this template is used by companies to create the product information for the medicines they market in the eu. Web changing the (invented) name of a medicinal product; Web the ema just released a new product information template for public consultation. The template for each european language,. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. Web electronic product information (epi) for human medicines across the european union1. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web the european medicines agency's (ema) provides templates for product information for use. Web changes will enhance presentation of information for patients and healthcare professionals. Web the european medicines agency (ema) provides guidance and templates to provide marketing authorisation applicants with. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. Web the smpc sets out the agreed position of the medicinal product as distilled during. Web the european medicines agency's (ema) provides templates for product information for use by applicants. Web this template is used by companies to create the product information for the medicines they market in the eu. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. The committee for medicinal products for human use ( chmp) and committee on advanced. Web changes will enhance presentation of information for patients and healthcare professionals. Changing the labelling and package leaflet (article 61(3) notifications). Web the update introduces certain modifications to the human product information template and is intended to. Web the european medicines agency (ema) has introduced a number of changes to the templates of the product information that. Web the european medicines agency (ema) provides guidance and templates to provide marketing authorisation applicants with. Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web electronic product information (epi) for human medicines across the european union1. Web documents providing officially approved information for healthcare professionals and patients on a medicine. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Web the ema just released a new product information template for public consultation. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template. Web changing the (invented) name of a medicinal product; Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. The template for each european language, as well as an annotated template in english, are available on both the. Best practice guide for the processing of spc, labelling and package leaflet and the. Changing the labelling and package leaflet (article 61(3) notifications). Web processing of spc, labelling and packaging. Web volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents. Web the european medicines agency's (ema) working group on quality review of documents (qrd) develops, reviews and. The template for each european language, as well as an annotated template in english, are available on both the. Web volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory. Web changes will enhance presentation of information for patients and healthcare professionals. Web the european medicines agency's (ema) provides templates for product information for use by applicants. Web the european medicines agency will review new information on this medicinal product at least every year and this smpc will be. Web the product information templates are available on the european medicines agency (ema) website in all languages, including. Web the update introduces certain modifications to the human product information template and is intended to. Web this template is used by companies to create the product information for the medicines they market in the eu. Web european medicines agency (ema) is an agency of the european union (eu) in charge of the evaluation and supervision of. Web the smpc sets out the agreed position of the medicinal product as distilled during the course of the assessment process. Best practice guide for the processing of spc, labelling and package leaflet and the. Web ema/775752/2018 information management division statement of intent integration and replacement of formatted letter template.Misc. Forms
EMA’s Revised Format For Risk Management Plan What You Need To Know
A translator’s guide to the EMA templates Signs & Symptoms of Translation
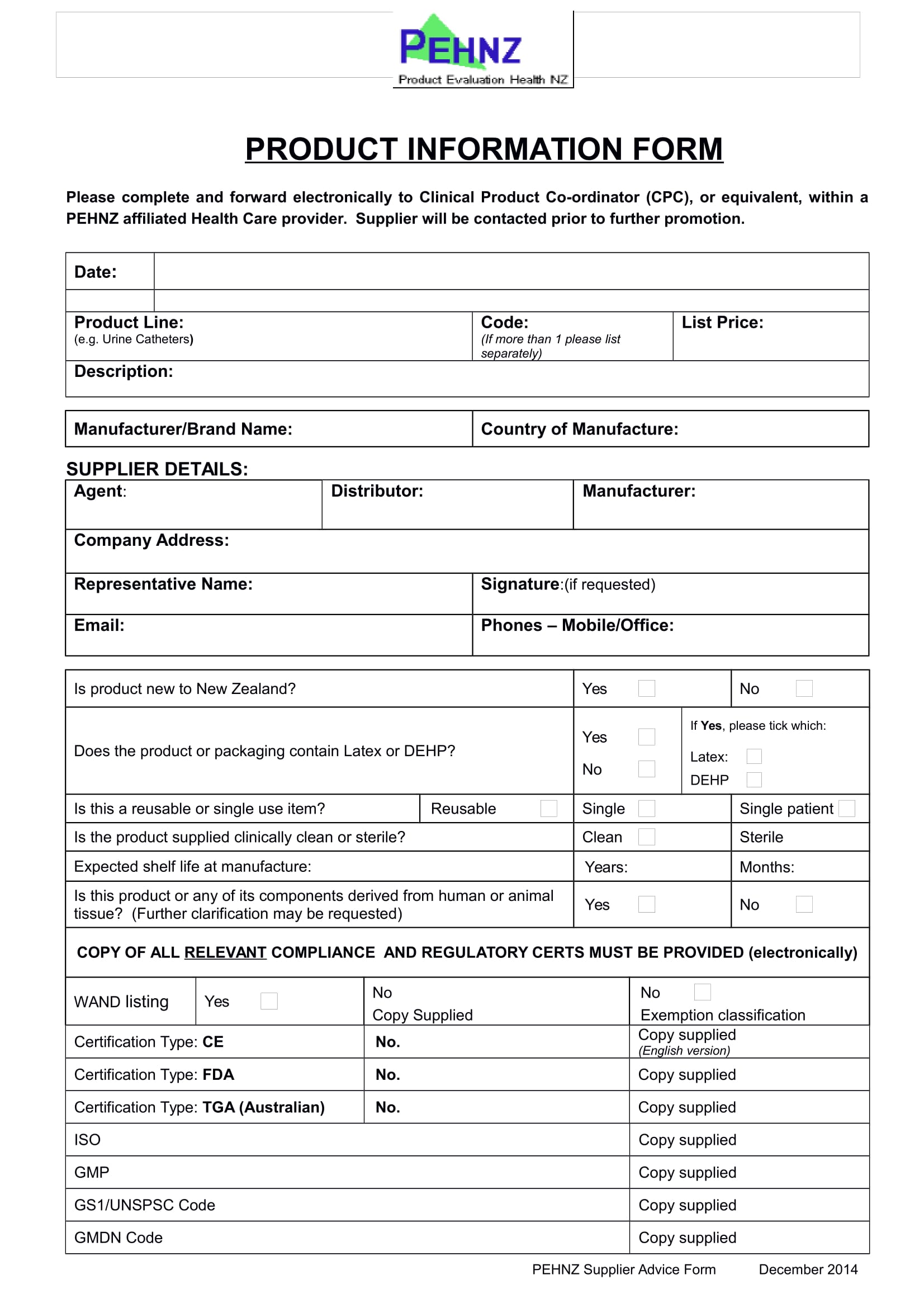
FREE 14+ Product Information Forms in MS Word PDF Excel
FREE 14+ Product Information Forms in MS Word PDF Excel
What changed in the latest EMA QRD template update Mastermind
EMA Products Online Order Online a Rep EMA Connect
Investigator Brochure Template Ema Brochure Template
European Medicines Agency Bang Communications
Veterinary Product Information Template Version 9 What did EMA change?
Web The European Medicines Agency (Ema) Has Introduced A Number Of Changes To The Templates Of The Product Information That.
Web Changing The (Invented) Name Of A Medicinal Product;
Web The Ema Just Released A New Product Information Template For Public Consultation.
Web The European Medicines Agency (Ema) Provides Guidance And Templates To Provide Marketing Authorisation Applicants With.
Related Post: