Data Management Plan Template Clinical Trial
Data Management Plan Template Clinical Trial - Clinical and/or mri data from human research participants. Data handling study team agreement. Web analysis, data management and clinical assessment operate together, identifying and removing risk and enabling quality from a. Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? Web august 7, 2023. Web clinical research budget plan template; Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Web sample plan a. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Web society for clinical data management (scdm) Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Clinical and/or mri data from human research participants. Web monthly with members of the project team, dr. Web august 7, 2023. Web society for clinical data management (scdm) Doppler (university of city, clinical imaging data collection), mr. Web sample plan a. Data handling study team agreement. Clinical data is either collected during patient care or as part of a clinical trial program. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Head of data management, norwich clinical trials unit, uea approved by: Web data management plan template. Web the sample data management and sharing plan below is for a proposal conducting clinical research with human. Web society for clinical data management (scdm) Head of data management, norwich clinical trials unit, uea approved by: Web society for clinical data management (scdm) Data collection and documentation 1.1 what data will you collect, observe, generate or reuse ? Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Web the purpose of this sop is to provide the. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web in an effort to minimise such risks and to establish a consistent approach, this document has been developed to cover. Clinical data is either collected during patient care or as part of a clinical trial program. Head of data management, norwich clinical trials unit, uea approved by: Clinical. Web 11 rows templates for data management plans are based on the specific requirements listed in funder policy. 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Web in an effort to minimise such risks and to establish a consistent approach, this document has been developed to cover. Web this data management plan template provides the required contents of a standard clinical trial data. Web data management plan template. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Clinical and/or mri data from human research participants. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web august 7, 2023. Web this data management plan template provides the required contents of a standard clinical trial data management. Doppler (university of city, clinical imaging data collection), mr. Clinical and/or mri data from human research participants. Head of data management, norwich clinical trials unit, uea approved by: Web this template is created based on experience within the association of clinical data managers. Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Clinical and/or mri data from human research participants. Data handling study team agreement. Web august 7, 2023. Clinical data is either collected during patient care or as part of a clinical trial program. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web analysis, data management and clinical assessment operate together, identifying and removing risk and enabling quality from a. Clinical. Web monthly with members of the project team, dr. Web introduction to clinical data. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during and after a research. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Clinical and/or mri data from human research participants. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Data handling study team agreement. Web this data management plan template provides the required contents of a standard clinical trial data management. Web 11 rows templates for data management plans are based on the specific requirements listed in funder policy. Web this template is created based on experience within the association of clinical data managers (acdm) and covers most. Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Web the sample data management and sharing plan below is for a proposal conducting clinical research with human. Head of data management, norwich clinical trials unit, uea approved by: Web data management plan template. Web in an effort to minimise such risks and to establish a consistent approach, this document has been developed to cover. Web clinical research budget plan template; 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Doppler (university of city, clinical imaging data collection), mr. Clinical data is either collected during patient care or as part of a clinical trial program. Rare disease patient, caregiver and advocate presentations from the fda’s “clinical. Web a data management plan, or dmp, is a formal document that outlines how data will be handled during and after a research. Clinical and/or mri data from human research participants. Web introduction to clinical data. Web august 7, 2023. 6é÷}ha 'wïö “ö®b å˜ à¤wúí+öëîfô™.sâx. Doppler (university of city, clinical imaging data collection), mr. Web the sample data management and sharing plan below is for a proposal conducting clinical research with human. Web dmptool is a fillable template that walks researchers through completing a plan that complies with funder. Web the purpose of this sop is to provide the minimum standards required to ensure all clinical trial data, from the point of. Web 11 rows templates for data management plans are based on the specific requirements listed in funder policy. Web data management plan template. Data handling study team agreement. Clinical research tracking log templates. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web analysis, data management and clinical assessment operate together, identifying and removing risk and enabling quality from a.All about Clinical Trial Data Management Smartsheet
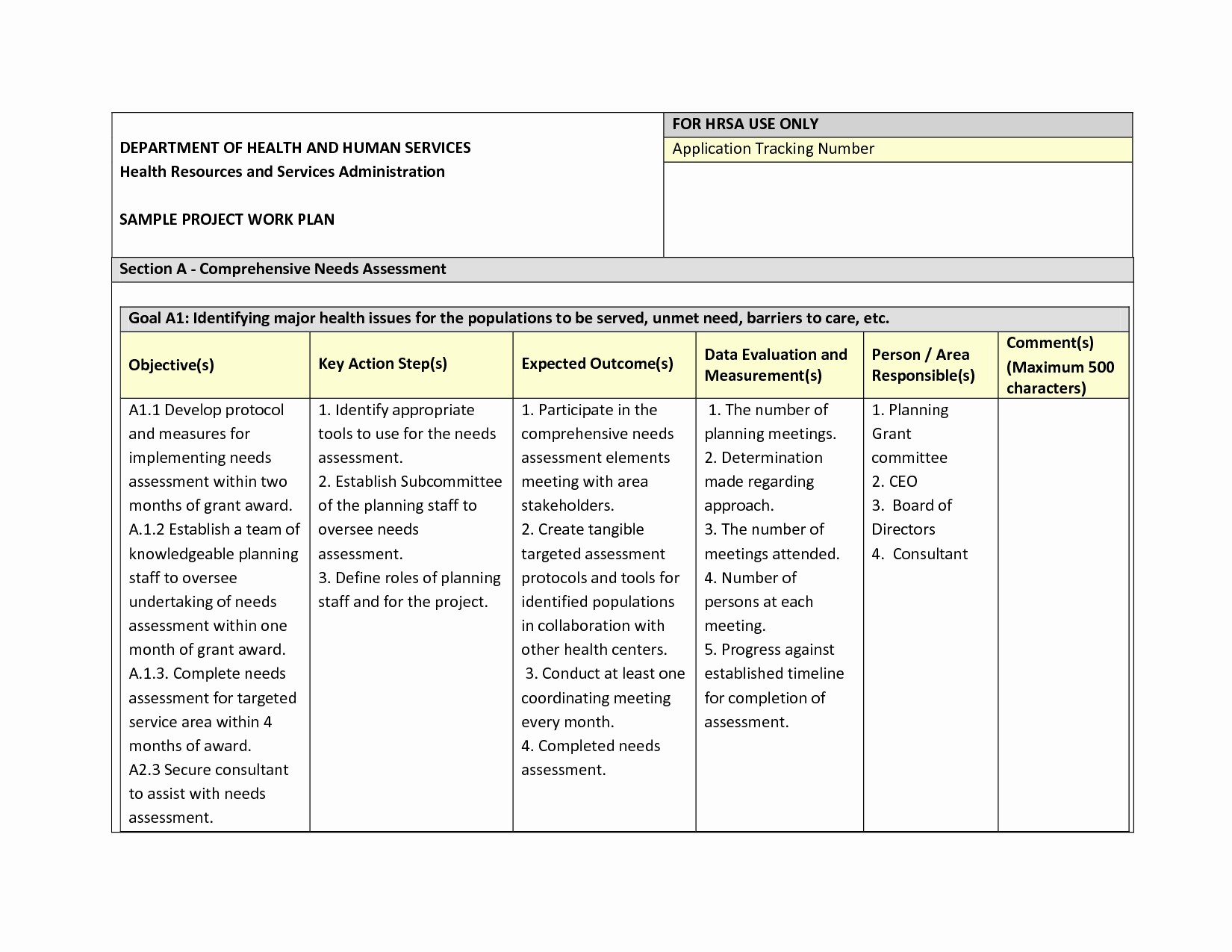
11+ Data Management Plan Examples PDF Examples
All about Clinical Trial Data Management Smartsheet
11+ Data Management Plan Examples PDF Examples
Free Clinical Trial Templates Smartsheet for Monitoring Report
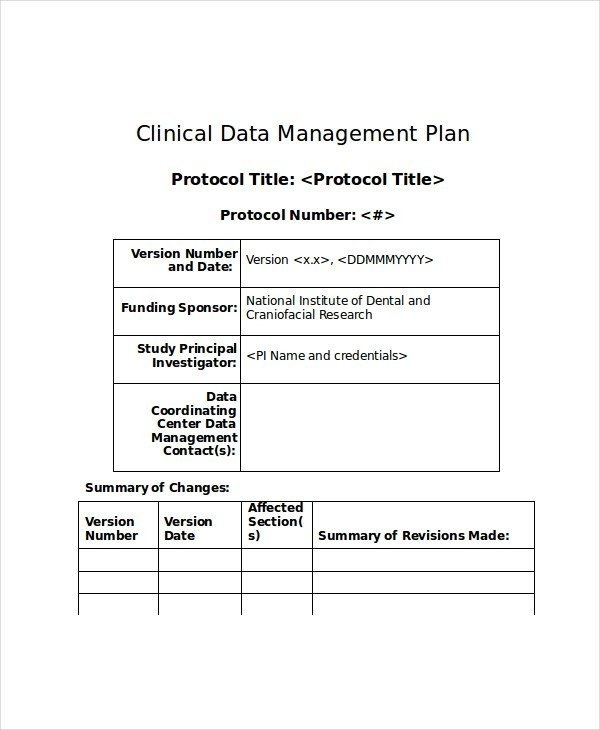
Data Management Plan Medical Research Council
Clinical Trial Report Template New Creative Template Ideas
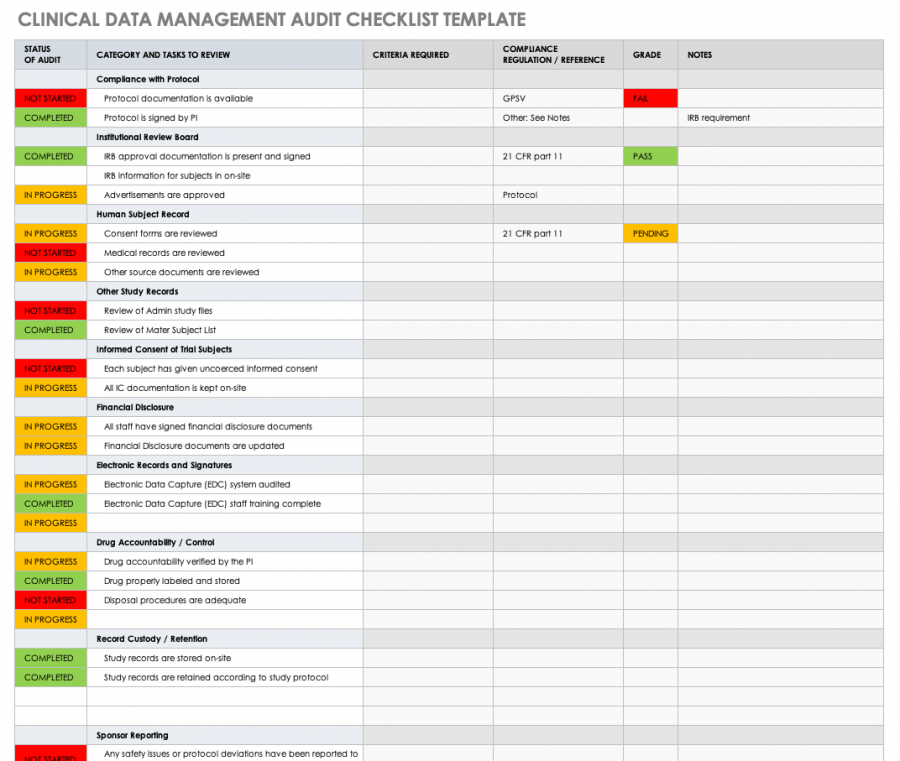
The enchanting The Basics Of Clinical Trial Centralized Monitoring For
All about Clinical Trial Data Management Smartsheet
Clinical Trial Template Master of Documents
Web Monthly With Members Of The Project Team, Dr.
Head Of Data Management, Norwich Clinical Trials Unit, Uea Approved By:
Web Sample Plan A.
Web Society For Clinical Data Management (Scdm)
Related Post: