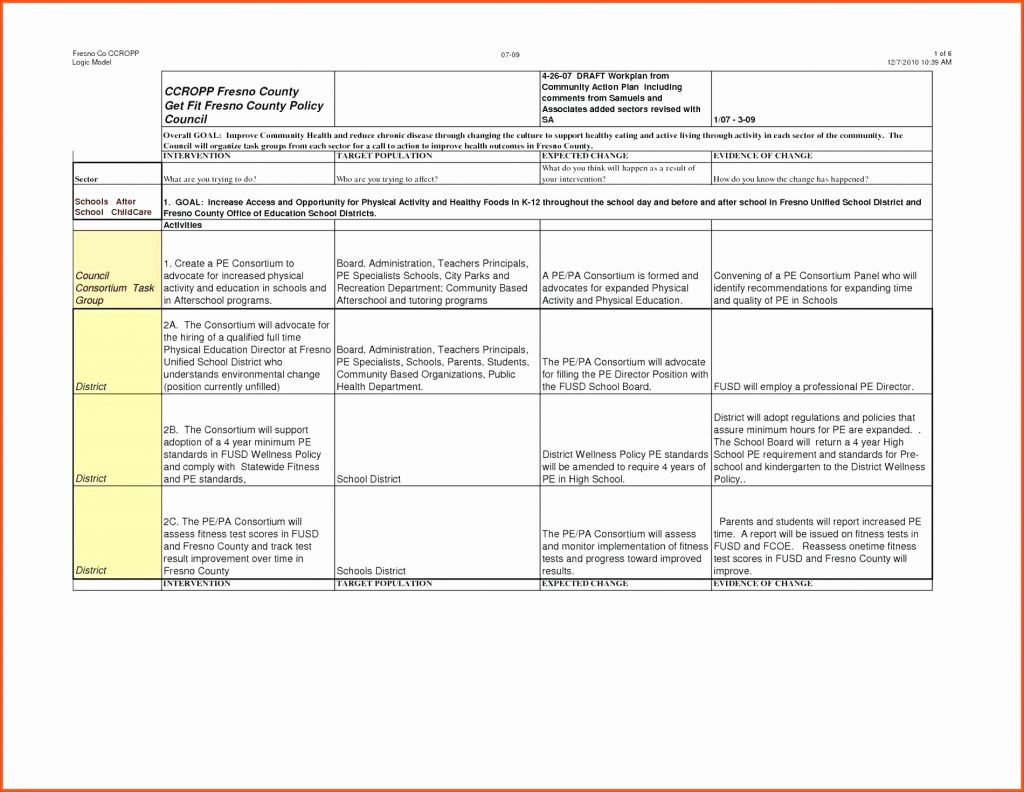
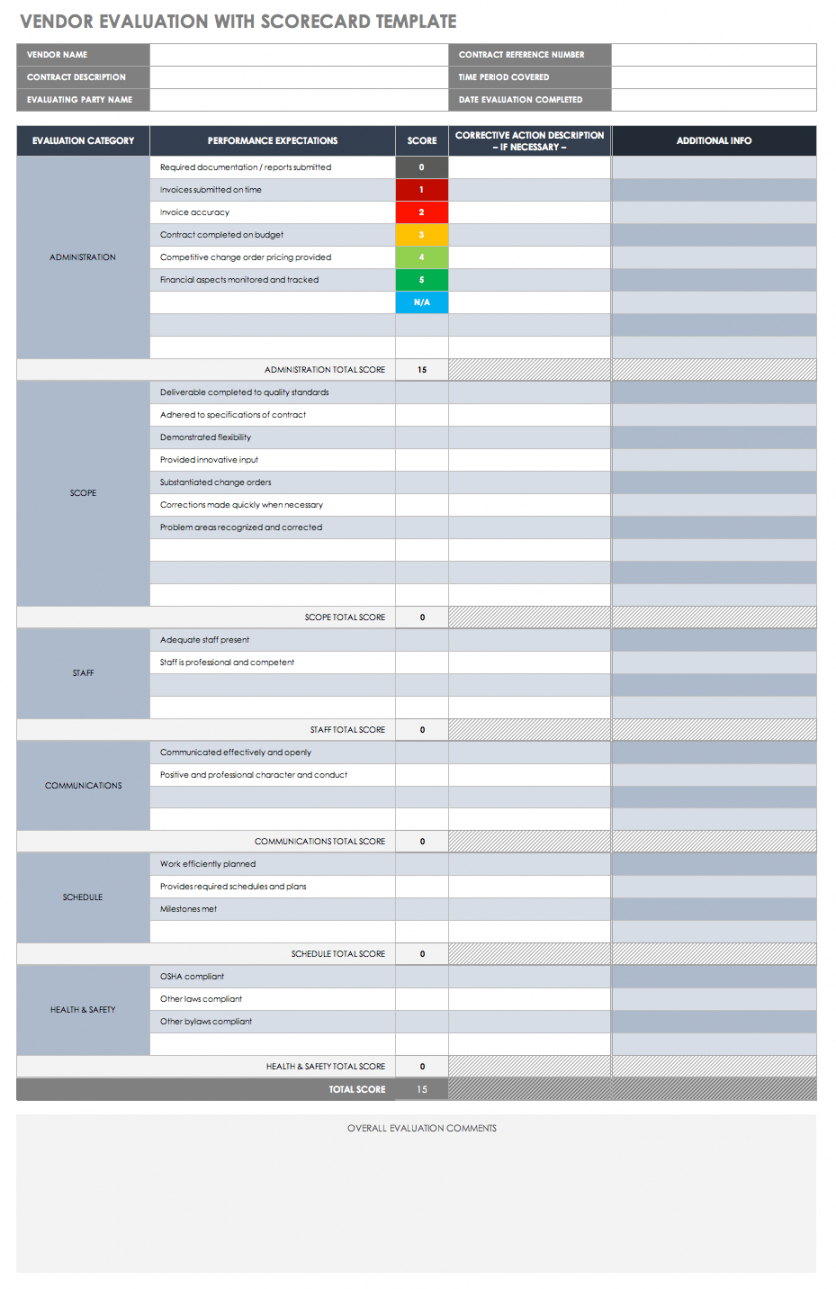
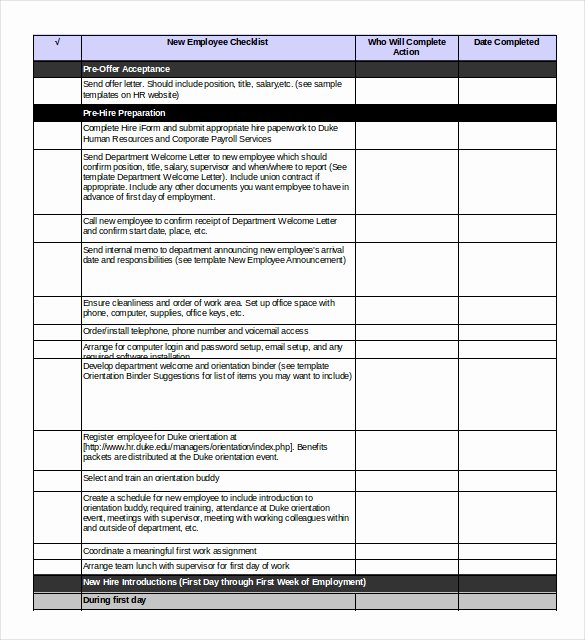
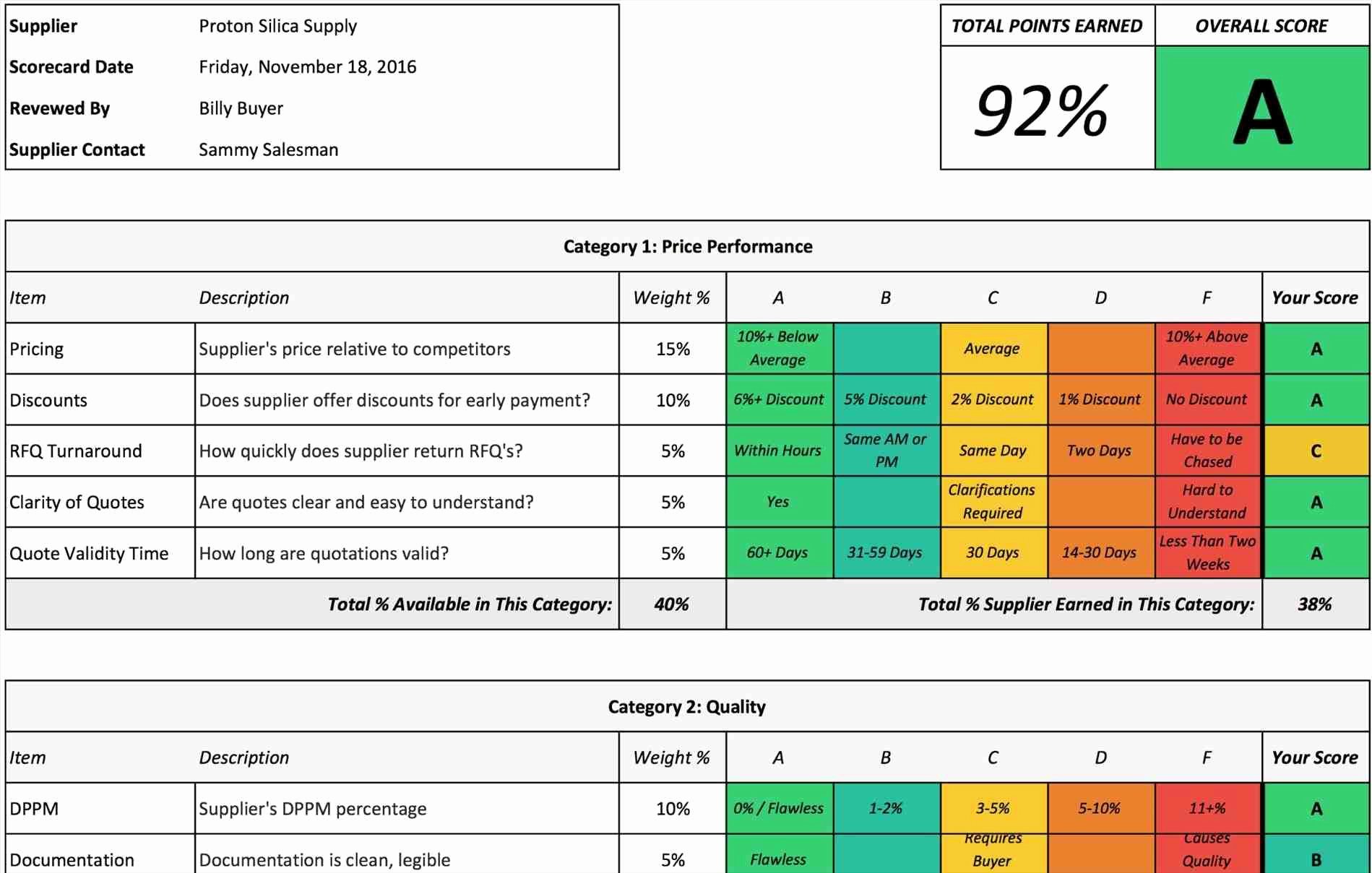
Vendor Management Plan Template Clinical Research
Vendor Management Plan Template Clinical Research - Web clinical study report template. Web learn how to manage clinical research vendors during which development process with this guide. Web a clinical quality agreement. Web download this white paper for practical insights and guidance on the key concepts that will help any sponsor, large, or small, better manage their. Web are your outsourcing practices integrated to gain the utmost efficiency and roi that it could be? Web liz wool, for wool consulting group, recommends so a vendor oversight plan template structure includes a cover. Web study how to manage clinical research vendors throughout the development proceed with dieser guide. Other elements include policies and. Web 1) identifying and selecting the right vendor. Web this white paper provides practical insights and guidance on the key concepts that will help any sponsor better. First things first, it’s vital to understand any prospective partner’s. Web a clinical quality agreement. Web are your outsourcing practices integrated to gain the utmost efficiency and roi that it could be? This is just an component of a value management system. Budget monitoring tool with example data. Web liz wool, for wool consulting group, recommends so a vendor oversight plan template structure includes a cover. Web this white paper provides practical insights and guidance on the key concepts that will help any sponsor better. Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Other elements include policies and. Web recent research. First things first, it’s vital to understand any prospective partner’s. Web 1) identifying and selecting the right vendor. Web recent research suggests that some pharmaceutical companies have found themselves out of compliance with ich,. Web clinical study report template. This is just an component of a value management system. First things first, it’s vital to understand any prospective partner’s. Web are your outsourcing practices integrated to gain the utmost efficiency and roi that it could be? Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Web learn how to manage clinical research vendors during which development process with this guide. Web an effective. Web recent research suggests that some pharmaceutical companies have found themselves out of compliance with ich,. Web society for clinical data management (scdm) Web creating a template in asana gives you the tools to streamline and scale your vendor management process, from sourcing and. Web study how to manage clinical research vendors throughout the development proceed with dieser guide. Web. Other elements include policies and. Web ccrps’ advanced clinical research project management certification (acrpmc) provides an excellent tool for. Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Web 1) identifying and selecting the right vendor. This is just an component of a value management system. Other elements include policies and. Web liz wool, for wool consulting group, recommends so a vendor oversight plan template structure includes a cover. First things first, it’s vital to understand any prospective partner’s. Budget monitoring tool with example data. Web are your outsourcing practices integrated to gain the utmost efficiency and roi that it could be? Web recent research suggests that some pharmaceutical companies have found themselves out of compliance with ich,. Use this request for proposal (rfp) with a. Web this white paper provides practical insights and guidance on the key concepts that will help any sponsor better. Budget monitoring tool with example data. Web are your outsourcing practices integrated to gain the utmost efficiency. Web creating a template in asana gives you the tools to streamline and scale your vendor management process, from sourcing and. Web 1) identifying and selecting the right vendor. First things first, it’s vital to understand any prospective partner’s. Web clinical study report template. Web the vendor oversight management plan (vomp) is a comprehensive document. Web download this white paper for practical insights and guidance on the key concepts that will help any sponsor, large, or small, better manage their. This is just an component of a value management system. Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Web 1) identifying and selecting the right vendor. Web society. Web society for clinical data management (scdm) Web study how to manage clinical research vendors throughout the development proceed with dieser guide. First things first, it’s vital to understand any prospective partner’s. Web liz wool, for wool consulting group, recommends so a vendor oversight plan template structure includes a cover. Web clinical study report template. Web learn how to manage clinical research vendors during which development process with this guide. Web creating a template in asana gives you the tools to streamline and scale your vendor management process, from sourcing and. Web 1) identifying and selecting the right vendor. This is just an component of a value management system. Budget monitoring tool with example data. Web recent research suggests that some pharmaceutical companies have found themselves out of compliance with ich,. Web download this white paper for practical insights and guidance on the key concepts that will help any sponsor, large, or small, better manage their. Web an effective vendor risk management program can help prevent costly delays and rework while enabling. Use this request for proposal (rfp) with a. Web ccrps’ advanced clinical research project management certification (acrpmc) provides an excellent tool for. Other elements include policies and. Web this white paper provides practical insights and guidance on the key concepts that will help any sponsor better. Web are your outsourcing practices integrated to gain the utmost efficiency and roi that it could be? Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Web a clinical quality agreement. Web society for clinical data management (scdm) Web creating a template in asana gives you the tools to streamline and scale your vendor management process, from sourcing and. Web free vendor management templates, such as contracts, scorecards, and vendor listings, are available for. Web the vendor oversight management plan (vomp) is a comprehensive document. Budget monitoring tool with example data. Web study how to manage clinical research vendors throughout the development proceed with dieser guide. Web liz wool, for wool consulting group, recommends so a vendor oversight plan template structure includes a cover. Web a clinical quality agreement. Web clinical study report template. Web 1) identifying and selecting the right vendor. Web recent research suggests that some pharmaceutical companies have found themselves out of compliance with ich,. Other elements include policies and. First things first, it’s vital to understand any prospective partner’s. Web this white paper provides practical insights and guidance on the key concepts that will help any sponsor better. Web ccrps’ advanced clinical research project management certification (acrpmc) provides an excellent tool for. This is just an component of a value management system.Vendor Management Checklist Template Awesome Management Review and
Vendor Spreadsheet pertaining to Sample Due Diligence Report Gallery Of
Printable Free Vendor Risk Assessment Templates Smartsheet Vendor
Vendor Management Excel Template Stcharleschill Template
Vendor Management Excel Template Stcharleschill Template
Vendor Risk Management Policy Template Template 1 Resume Examples
Editable Free Vendor Risk Assessment Templates Smartsheet Vendor
VENDOR MANAGEMENT SOP Template PH56 GMP, QSR & ISO Compliance
Vendor Risk assessment Template Stcharleschill Template
Editable Free Vendor Risk Assessment Templates Smartsheet Vendor
Web An Effective Vendor Risk Management Program Can Help Prevent Costly Delays And Rework While Enabling.
Web Are Your Outsourcing Practices Integrated To Gain The Utmost Efficiency And Roi That It Could Be?
Web Learn How To Manage Clinical Research Vendors During Which Development Process With This Guide.
Use This Request For Proposal (Rfp) With A.
Related Post: