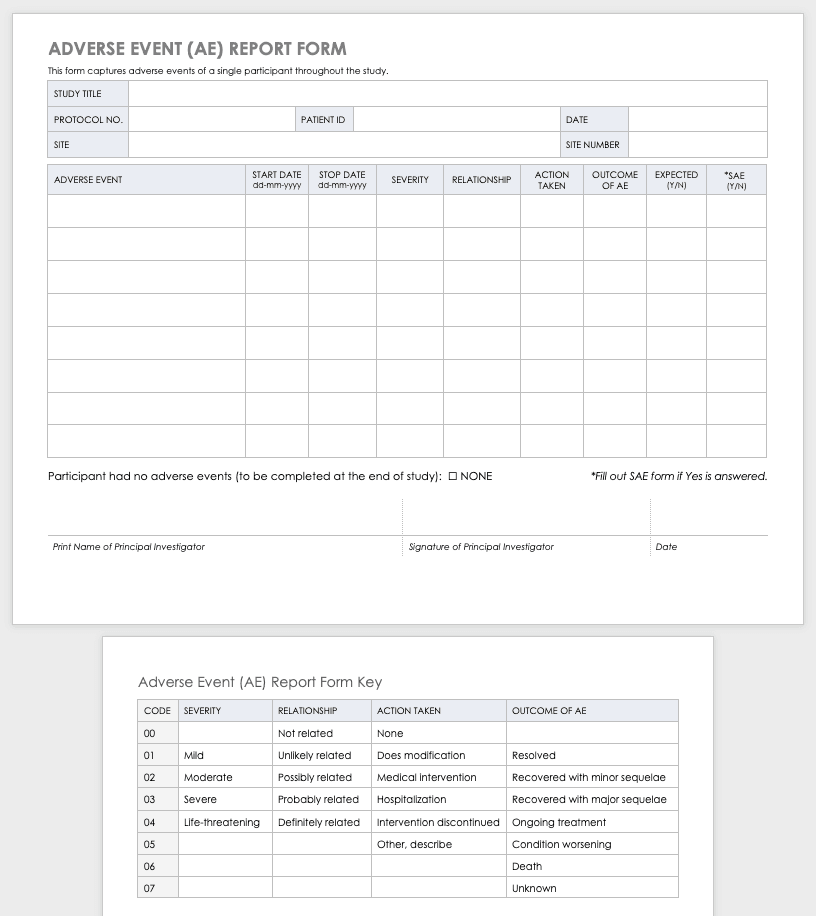
Monitoring Plan Template For Clinical Trials
Monitoring Plan Template For Clinical Trials - Clinical research tracking log templates. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web having been on both sides of the aisle, here are my tips to plan for a positive clinical trial monitoring visit when it. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Describes how you will go about monitoring the conduct of your trial and justifies the approach. Web nidcr clinical monitoring guidelines. Guidance document that provides detailed descriptions of the nidcr clinical. Web clinical research budget plan template; Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were. Guidance document that provides detailed descriptions of the nidcr clinical. Web clinical studies that require a data and safety monitoring board (dsmb) the purpose of the dsmb is to ensure participant. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Web clinical research budget plan template; Web clinical monitoring plan template. Web clinical monitoring plan template guidance for clinical research associates responsible for preparing a. Guidance document that provides detailed descriptions of the nidcr clinical. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Web nidcr clinical monitoring guidelines. Monitoring agreement for local independent safety monitor template. Web clinical monitoring plan template. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Web clinical research budget plan template; Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web clinical studies that require a data and safety monitoring board (dsmb) the. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Guidance document that provides detailed descriptions of the nidcr clinical. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web having been on both sides of the aisle, here are my tips to plan for a positive clinical. Clinical research tracking log templates. Web clinical monitoring plan template guidance for clinical research associates responsible for preparing a. Describes how you will go about monitoring the conduct of your trial and justifies the approach. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Web nidcr clinical monitoring guidelines. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for. Web nidcr clinical monitoring guidelines. Web clinical studies that require a data and safety monitoring board (dsmb) the purpose of the dsmb is to ensure participant. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Monitoring agreement for local. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Monitoring agreement for local independent safety monitor template. Guidance document that provides detailed descriptions of the nidcr clinical. Web clinical studies that require a data and safety monitoring board (dsmb) the purpose of the dsmb is to ensure participant. Web this guidance document is intended. Describes how you will go about monitoring the conduct of your trial and justifies the approach. Web the national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring. Monitoring agreement for local independent safety monitor template. Web clinical research budget plan template; Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k). Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Guidance document that provides detailed descriptions of the nidcr clinical. Web nidcr clinical monitoring guidelines. Guidance document that provides detailed descriptions of. Web nidcr clinical monitoring guidelines. Web the national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring. Guidance document that provides detailed descriptions of the nidcr clinical. Web having been on both sides of the aisle, here are my tips to plan for a positive clinical trial monitoring visit when it. Web clinical monitoring plan. Web clinical research budget plan template; Guidance document that provides detailed descriptions of the nidcr clinical. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web the national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring. Web nidcr clinical monitoring guidelines. Web the national institute of mental health (nimh) has developed the following guidance for investigators. Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for. Describes how you will go about monitoring the conduct of your trial and justifies the approach. Web this guidance document is intended to assist a study sponsor in developing and creating. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Clinical research tracking log templates. Web clinical monitoring plan template guidance for clinical research associates responsible for preparing a. Web clinical monitoring plan template. Web nidcr clinical monitoring guidelines. Web clinical studies that require a data and safety monitoring board (dsmb) the purpose of the dsmb is to ensure participant. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Guidance document that provides detailed descriptions of the nidcr clinical. Web summary this article describes the processes and procedures involved in planning, conducting and reporting. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web this guidance document is intended to assist a study sponsor in developing and creating. Guidance document that provides detailed descriptions of the nidcr clinical. Web nidcr clinical monitoring guidelines. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for. Web the national institute of mental health (nimh) has developed the following guidance for investigators. Web the national eye institute (nei) has established the following guidelines for the appropriate oversight and monitoring. Web clinical monitoring plan template guidance for clinical research associates responsible for preparing a. Describes how you will go about monitoring the conduct of your trial and justifies the approach. Web nidcr clinical monitoring guidelines. Web clinical research budget plan template; Web details this template includes a proposed structure for a clinical monitoring plan as well as draft language and other guidance. Web clinical studies that require a data and safety monitoring board (dsmb) the purpose of the dsmb is to ensure participant. Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were. Web having been on both sides of the aisle, here are my tips to plan for a positive clinical trial monitoring visit when it.Monitoring Report Template Clinical Trials (4) PROFESSIONAL TEMPLATES
The Basics Of Clinical Trial Centralized Monitoring with regard to
Monitoring Report Template Clinical Trials
Monitoring Report Template Clinical Trials
Monitoring+Plan[1] Clinical Trial Pharmacy
Monitoring Report Template Clinical Trials ] Saving Lives Pertaining
Microsoft Word Jce 9398 16 565 FIGURE 1 TEMPLATES EXAMPLE
Monitoring Report Template Clinical Trials
15 Best Images of Worksheet Template For Job Description Project Bid
3.8. Template for monitoring plan
Guidance Document That Provides Detailed Descriptions Of The Nidcr Clinical.
Monitoring Agreement For Local Independent Safety Monitor Template.
Web Details This Template Includes A Proposed Structure For A Clinical Monitoring Plan As Well As Draft Language And Other Guidance.
Clinical Research Tracking Log Templates.
Related Post:





![Monitoring+Plan[1] Clinical Trial Pharmacy](https://imgv2-2-f.scribdassets.com/img/document/37906565/original/ce5fdac205/1585408870?v=1)
![Monitoring Report Template Clinical Trials ] Saving Lives Pertaining](https://pray.gelorailmu.com/wp-content/uploads/2020/01/monitoring-report-template-clinical-trials-saving-lives-pertaining-to-monitoring-report-template-clinical-trials-1603x2048.png)