Medical Device Quality Plan Template
Medical Device Quality Plan Template - Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. Web 1 medical device quality agreement template prepared by dan o'leary ombu enterprises, llc. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and. The document should be tailored to the specific. Medical device description and specification. These procedures were written by quality professionals from. Web med dev qms templates are proven procedures that are efficient and easy to understand. Web iso 13485 document template: Make a monthly sale of about $450,000 and about $950,000 for the first. Web iso 13485 templates. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso. Web. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. Web 1 medical device quality agreement template prepared by dan o'leary ombu enterprises, llc. The document should be tailored to the specific. Web here we describe the. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. The iso 13485 is the standard for quality management in the medical. Click here for a medical device project plan you can use to keep your project on track and running. 24, 2021 • iso 13485, regulation (eu) 2017/745 the european regulation for medical devices requires.. Web quality plans are documented plans that outline quality policies, procedures, practices, and guidelines for. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. Click here for a medical device project plan you can use to keep. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Quality plan the quality plan is the documented list of arrangements needed for the creation of. 24, 2021 •. Web a medical device quality management system (qms) is a structured system that documents the procedures. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. 24, 2021 • iso 13485, regulation (eu) 2017/745 the european regulation for medical devices requires. Web this article explains how to write a quality system plan template. Web this document is intended to form the basis for a supplier agreement for a medical device manufacturer. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. Web a medical device quality management system (qms) is a structured system that documents the procedures. Web a library of free medical device templates and. Web med dev qms templates are proven procedures that are efficient and easy to understand. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web iso 13485 document template: Click here for a medical device project plan you can use to keep your project on track and running. Web quality plans are documented plans that. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; The document should be tailored to the specific. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. The iso 13485 is the standard for quality management in. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web 1 medical device quality agreement template prepared by dan o'leary ombu enterprises, llc. Click here for a medical device project plan you can use to keep your project on track and running. The document should be tailored to the specific. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso. Web crafting a quality planner for medical device companies is difficult, and it must contain these 13 essentials if you. Web this medical devices development plan describes in detail all essential steps to be considered prior to start the development. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. Web quality plans are documented plans that outline quality policies, procedures, practices, and guidelines for. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and. 24, 2021 • iso 13485, regulation (eu) 2017/745 the european regulation for medical devices requires. Web a medical device quality management system (qms) is a structured system that documents the procedures. The iso 13485 is the standard for quality management in the medical. Web this document is intended to form the basis for a supplier agreement for a medical device manufacturer. When building a quality assurance plan, adhering to regulatory requirements should be your. Web this article outlines an eu mdr quality plan for compliance with european regulation 2017/745 for medical. Web in the intricate landscape of medical device development, ensuring patient safety is paramount. Web sell a minimum of 5000 medical devices per day globally. These procedures were written by quality professionals from. Web a medical device project plan template can help streamline all the important steps involved in creating, designing, and. Web sell a minimum of 5000 medical devices per day globally. Web med dev qms templates are proven procedures that are efficient and easy to understand. The iso 13485 is the standard for quality management in the medical. However it is an essential tool that can help medical device manufacturer to deliver on specific projects. In general, quality plan is not a specific requirements from the iso 13485:2016 or from fda quality system regulation 21 cfr 820; Web quality plans are documented plans that outline quality policies, procedures, practices, and guidelines for. Quality plan the quality plan is the documented list of arrangements needed for the creation of. Web this article explains how to write a quality system plan template to revise and update your quality system for compliance with iso. Click here for a medical device project plan you can use to keep your project on track and running. Web design and development plan template (medical device per iso 13485 and 21 cfr 820) free 0 € (ex. Web here we describe the 10 essential contents of a quality plan, according to iso 10005:2018. When building a quality assurance plan, adhering to regulatory requirements should be your. The document should be tailored to the specific. These procedures were written by quality professionals from. Medical device description and specification.Md 001medicaldevicesdesigncontrolsop1.022
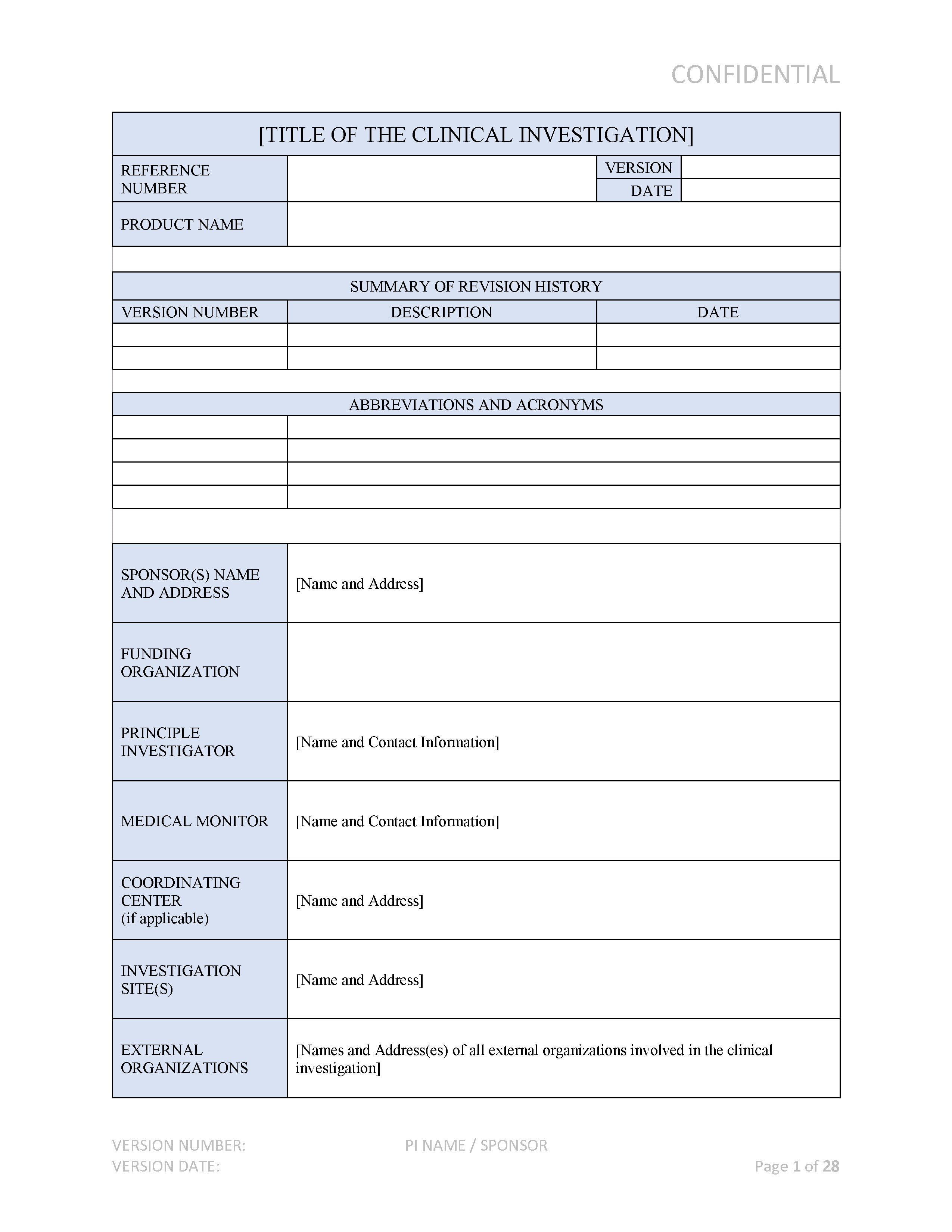
Medical Device Clinical Investigation Plan (CIP) ISO 141552020 Compliant
Medical Device Quality Management System DESIGN PLUS
Medical Device Quality Agreement US
Addictionary
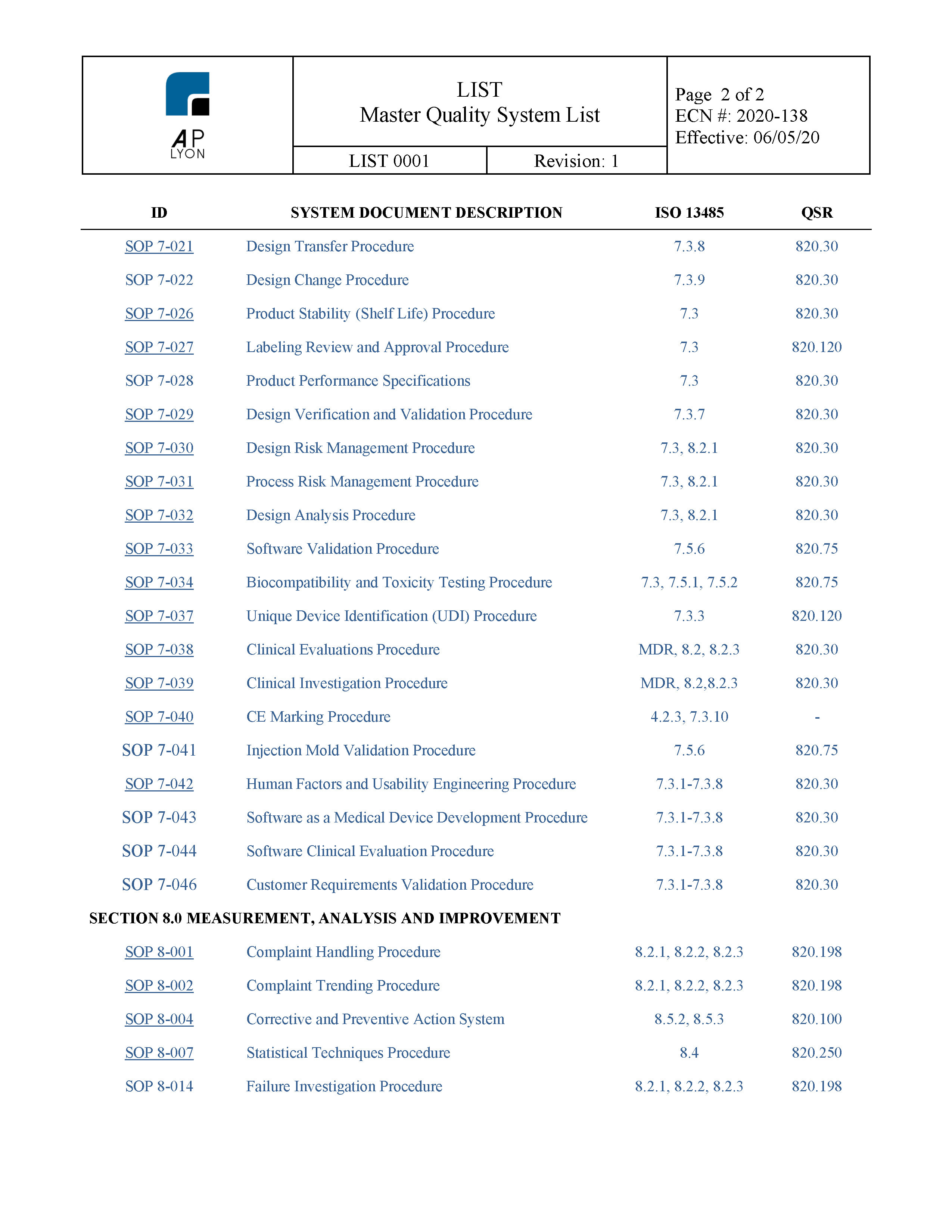
Supplier Quality Manual Template Get Free Templates
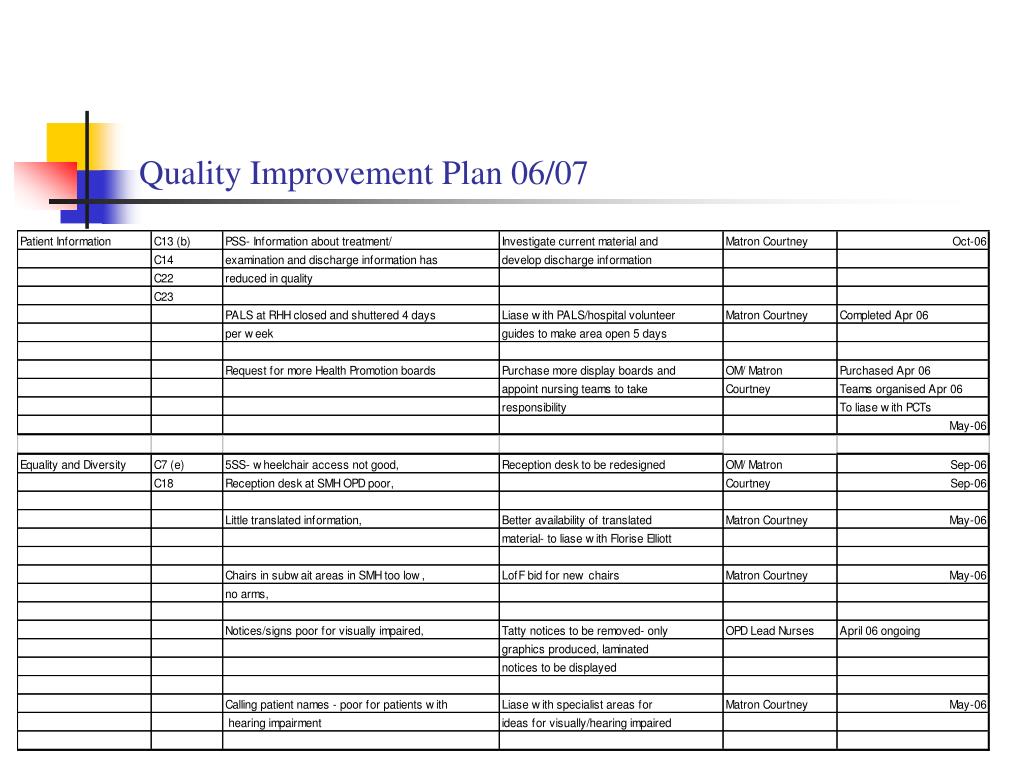
PPT Listening to Patients. OUTPATIENT QUALITY IMPROVEMENT PLAN 06/07
Installation Qualification IQ Procedure
Calibration System Procedure
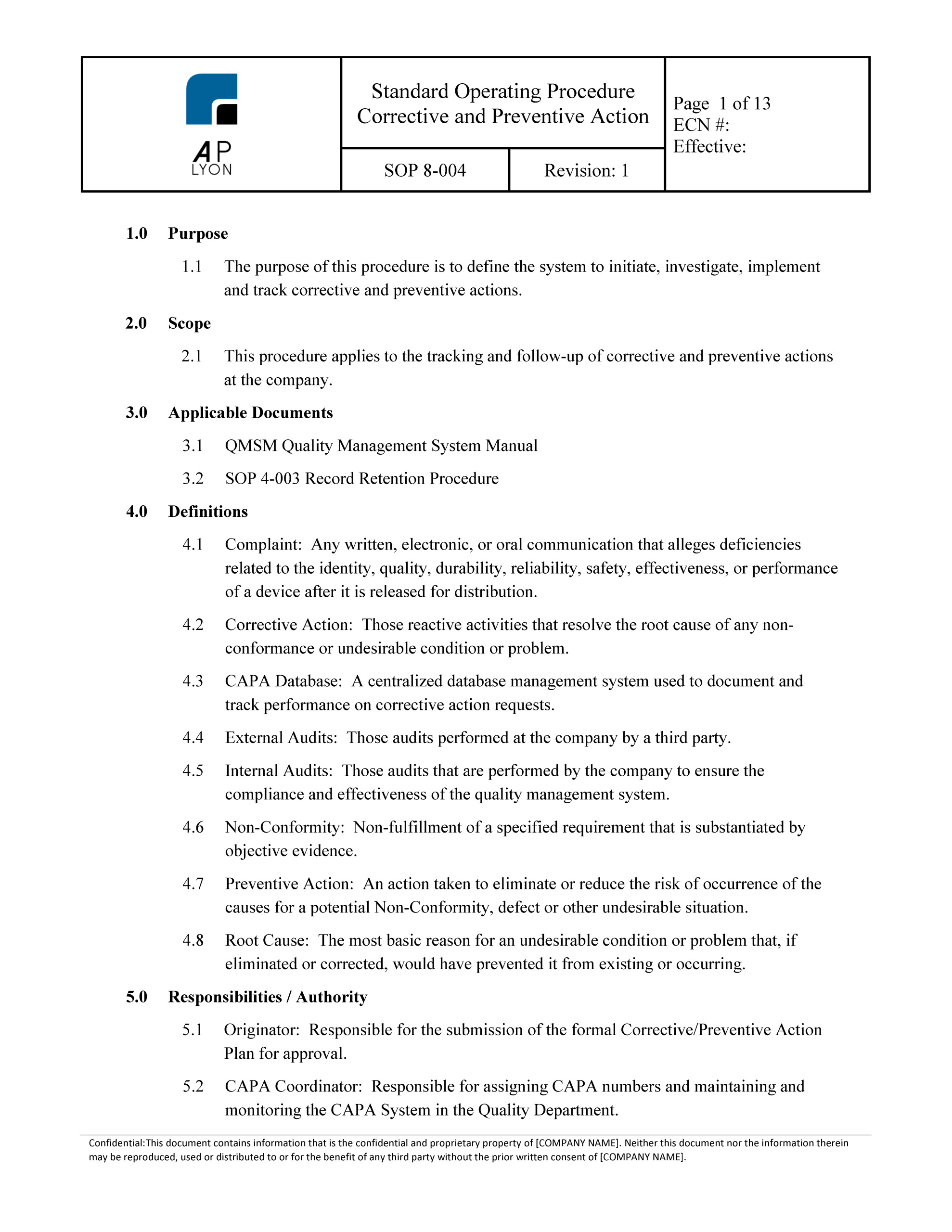
Corrective and Preventive Action Procedure
24, 2021 • Iso 13485, Regulation (Eu) 2017/745 The European Regulation For Medical Devices Requires.
Web Iso 13485 Templates.
Web Crafting A Quality Planner For Medical Device Companies Is Difficult, And It Must Contain These 13 Essentials If You.
Web A Medical Device Quality Management System (Qms) Is A Structured System That Documents The Procedures.
Related Post: