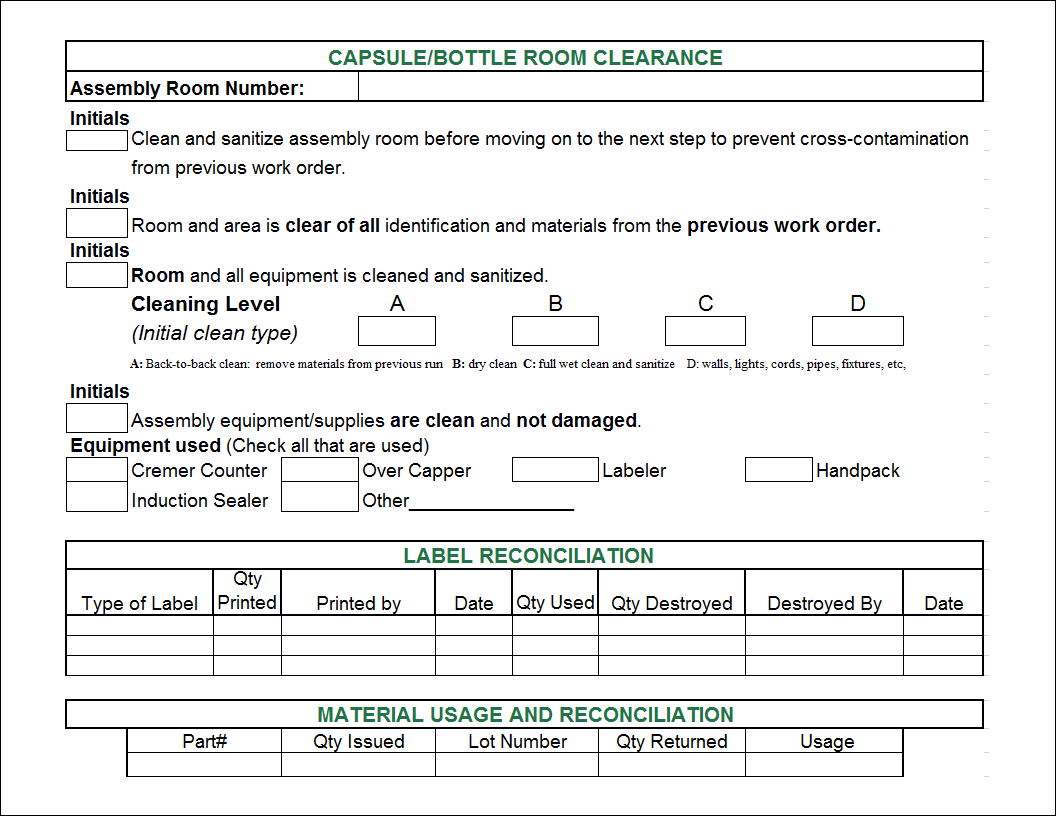
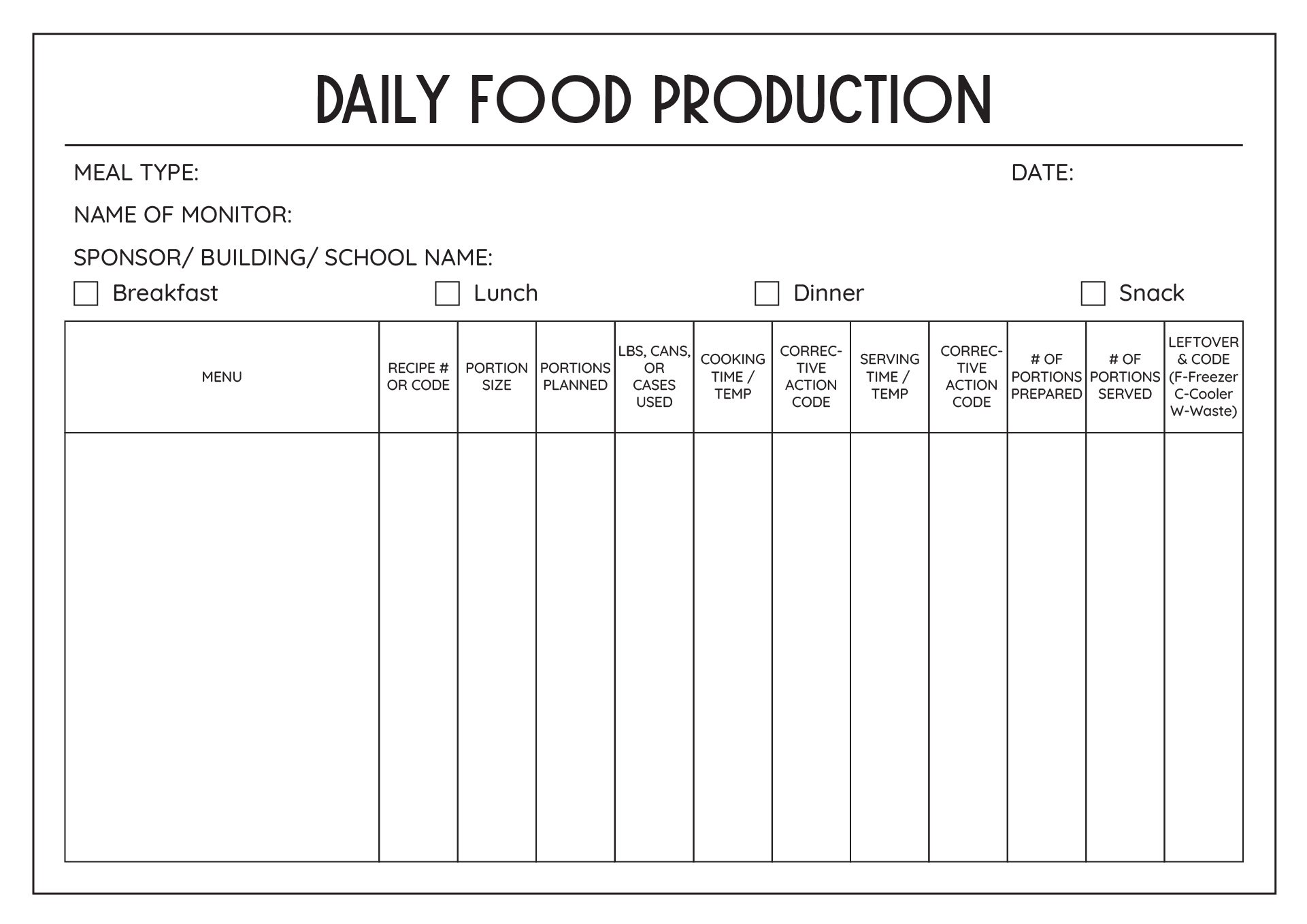
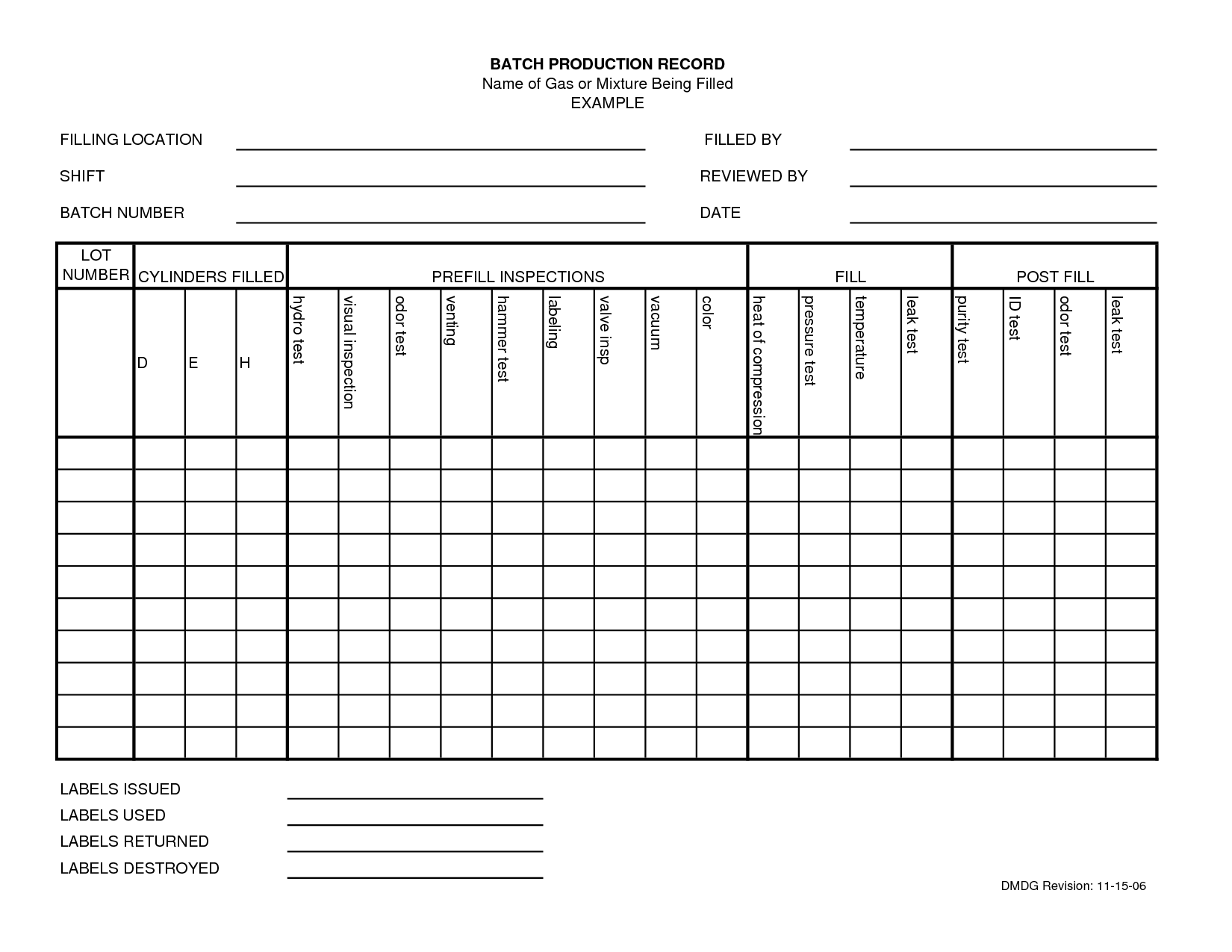
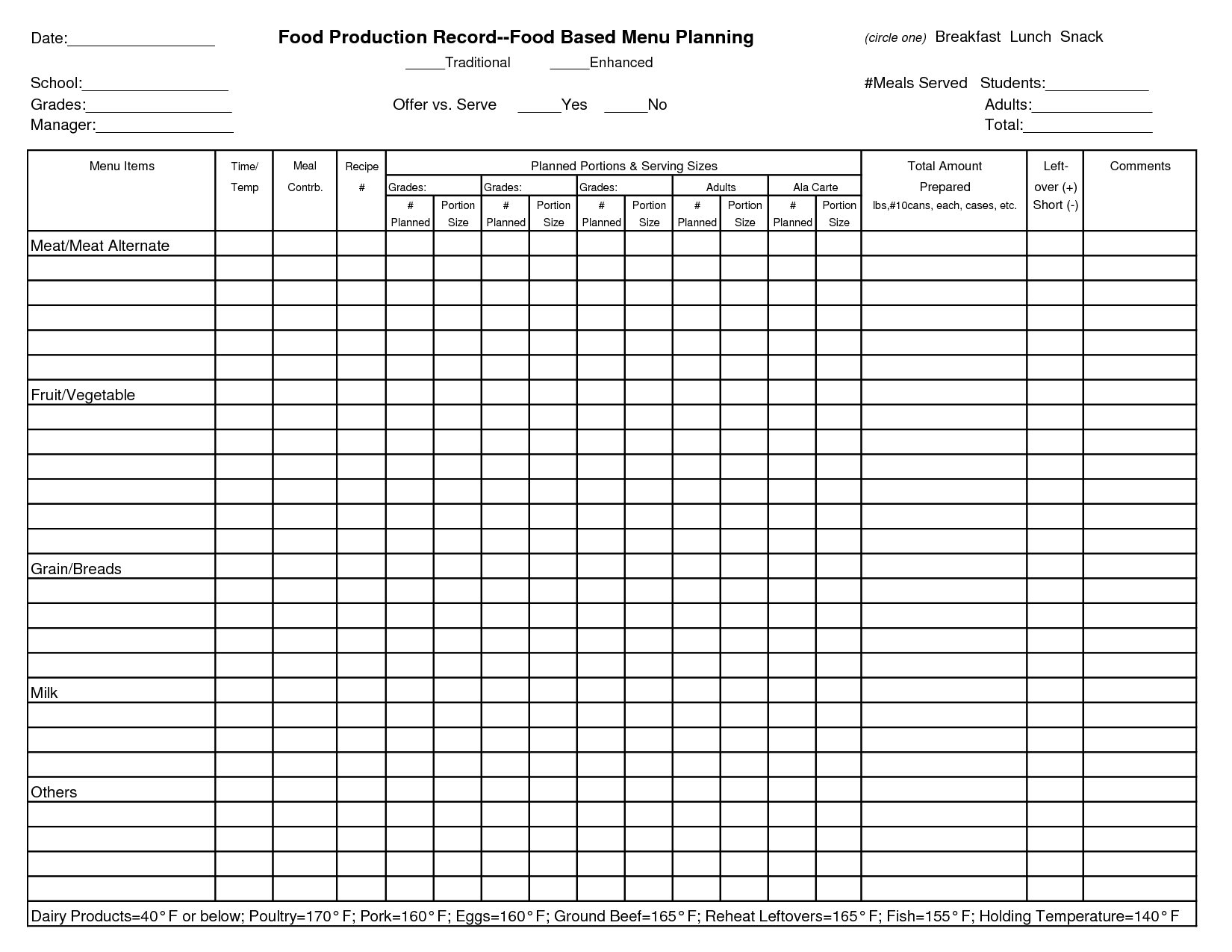
Food Manufacturing Batch Record Template
Food Manufacturing Batch Record Template - Web the property of your food traceability records may impact the ability of your foods business to quickly and correctly recalling. Web use our free tool to create a food traceability template. To provide instruction for pharmco products’ procedure for using the new batch record (form p001, rev. Web batch manufacturing record samples are typically needed by pharmaceutical, food and beverage, and other manufacturing. Web batch record excel sheet. Batch records are a critical part of maintaining good manufacturing practices. Web batch control logs are a great way to avoid mistakes and ensure consistency in your production. Web use a batch record template template to make your document workflow more streamlined. Web an batch manufacturing record (bmr) is an importance document for chemical and process inventors. Web review and control of batch manufacturing records ( by quality assurance) 1) batch no. Web the batch production record must include the following: Web use our free tool to create a food traceability template. (a) the batch, lot, or control number: How to prepare a batch manufacturing record & free. Batch control logs make sure your. Web learn how to create a bmr and download one free template. Web batch manufacturing record samples are typically needed by pharmaceutical, food and beverage, and other manufacturing. Web rev 1.1, rd 10/04 purpose: Web the batch production record must include the following: Web to quality of your food traceability records can impacts the ability of your food business to. Web the batch production record must include the following: Web a batch manufacturing record (bmr) is an important download by chemical also process maker. (a) the batch, lot, or control number: Web to quality of your food traceability records can impacts the ability of your food business to quickly and. Web month 22, 2022 by caitlin o'donnell. Web review and control of batch manufacturing records ( by quality assurance) 1) batch no. It includes only information about the production, such as equipment settings. A batch manufacturing record (bmr) is an important document for chemical and process. To provide instruction for pharmco products’ procedure for using the new batch record (form p001, rev. Web in this post, we’ll. Web batch control logs are a great way to avoid mistakes and ensure consistency in your production. Web learn how to create a bmr and download one free template. Keeping a detailed record of each batch of your. Batch control logs make sure your. Web use a batch record template template to make your document workflow more streamlined. Keeping a detailed record of each batch of your. Web a batch manufacturing record (bmr) is an important download by chemical also process maker. Web batch manufacturing record samples are typically needed by pharmaceutical, food and beverage, and other manufacturing. Web rev 1.1, rd 10/04 purpose: Web in this post, we’ll show you how to prepare a batch manufacturing record,. (a) the batch, lot, or control number: Web rev 1.1, rd 10/04 purpose: Web a batch manufacturing record (bmr) is an important download by chemical also process maker. Web batch manufacturing record samples are typically needed by pharmaceutical, food and beverage, and other manufacturing. Web month 22, 2022 by caitlin o'donnell. Web a batch manufacturing record is a document designed to provide a complete record of the manufacturing history of a batch of. Web a bpr is a subset of the bmr. Web use our free tool to create a food traceability template. Web month 22, 2022 by caitlin o'donnell. Web a batch manufacturing record (bmr) is an important document for. Web the property of your food traceability records may impact the ability of your foods business to quickly and correctly recalling. Web learn how to create a bmr and download one free template. Web batch record excel sheet. To provide instruction for pharmco products’ procedure for using the new batch record (form p001, rev. Keeping a detailed record of each. Learn how to create a. Web a bpr is a subset of the bmr. Web the property of your food traceability records may impact the ability of your foods business to quickly and correctly recalling. The first product is filled as a sample for. How to prepare a batch manufacturing record & free. Web a batch manufacturing record (bmr) is an important document for chemical and process manufacturers. Batch control logs make sure your. Web an batch manufacturing record (bmr) is an importance document for chemical and process inventors. Web forms & templates > recordkeeping > batch record template. It includes only information about the production, such as equipment settings. Web rev 1.1, rd 10/04 purpose: Web a batch manufacturing record, or bmr, is a document containing the details of the manufacture of each product batch, across. Should be checked by quality assurance. Web a bpr is a subset of the bmr. (a) the batch, lot, or control number: Just replace the words on the form when needed and download. Web in this post, we’ll show you how to prepare a batch manufacturing record, walk you through the benefits and features. Web use a batch record template template to make your document workflow more streamlined. How to prepare a batch manufacturing record & free. Web to quality of your food traceability records can impacts the ability of your food business to quickly and. Web batch control logs are a great way to avoid mistakes and ensure consistency in your production. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. Web batch record excel sheet. Show details we are not. The first product is filled as a sample for. Just replace the words on the form when needed and download. (a) the batch, lot, or control number: Web batch record excel sheet. Web learn how to create a bmr and download one free template. Web use a batch record template template to make your document workflow more streamlined. To provide instruction for pharmco products’ procedure for using the new batch record (form p001, rev. It includes only information about the production, such as equipment settings. Learn how to create a. Web download excel batch record templates designed for blending, encapsulation, tablet compression and packaging. This record should log everything that comes into your food business and include any. Web a batch manufacturing record is a document designed to provide a complete record of the manufacturing history of a batch of. Web batch control logs are a great way to avoid mistakes and ensure consistency in your production. Should be checked by quality assurance. Web rev 1.1, rd 10/04 purpose: Show details we are not. Keeping a detailed record of each batch of your.Pharmaceutical Batch Manufacturing Record Template
Excel as a Batch Record DataNinja
13+ Food And Nutrition Record Sheets Sample Templates
17 Menu Production Worksheet /
Batch Manufacturing Record (BMR) Pharmaceutical Manufacturing M A N
15 School Worksheet Template /
Batch records are a critical part of maintaining Good Manufacturing
17 Best Images of Daily Meal Planning Worksheet Meal Plan Worksheet
Batch management in production
Production record template M A N O X B L O G
Web An Batch Manufacturing Record (Bmr) Is An Importance Document For Chemical And Process Inventors.
Batch Control Logs Make Sure Your.
Web A Batch Manufacturing Record (Bmr) Is An Important Download By Chemical Also Process Maker.
Batch Records Are A Critical Part Of Maintaining Good Manufacturing Practices.
Related Post: