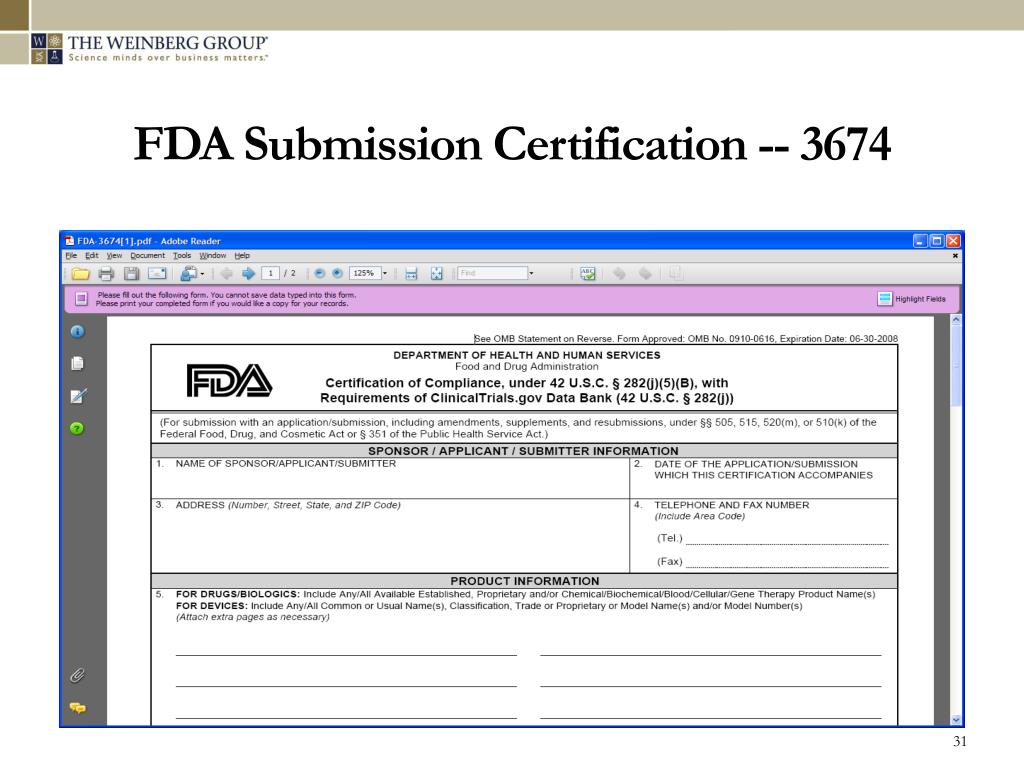
Fda Pre Submission Template
Fda Pre Submission Template - Web how to use the electronic submission template and resource (estar) pdf template. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. Web the estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical. The center for devices and radiological health (cdrh) accepts and encourages the. Electronic regulatory submission and review. Web clinical data for premarket submissions. Web the presubmission process can be used to get advice from fda on a range of issues associated with 510 (k), pma, ide,. Web electronic submission template for medical device 510(k) submissions guidance for industry and food and drug administration. Web an electronic copy (ecopy) is defined as an exact duplicate of the paper submission, created and submitted on a compact disc. Web regulatory resources template documents these template documents are meant to serve as a guide for preparation of. Web clinical data for premarket submissions. Web official fda applications and submissions forms. Web how to use the electronic submission template and resource (estar) pdf template. The center for devices and radiological health (cdrh) accepts and encourages the. The center for devices and radiological health (cdrh) accepts and encourages the. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. Web pre submission is a way for the industry to get the opinion from the fda before the submission of a premarket. Web an electronic copy (ecopy) is defined as. Web pre submission is a way for the industry to get the opinion from the fda before the submission of a premarket. The center for devices and radiological health (cdrh) accepts and encourages the. Web regulatory resources template documents these template documents are meant to serve as a guide for preparation of. Web the presubmission process can be used to. Electronic regulatory submission and review. Web pre submission is a way for the industry to get the opinion from the fda before the submission of a premarket. Web the estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical. Web an electronic copy (ecopy) is defined as an exact duplicate of the paper. Web an electronic copy (ecopy) is defined as an exact duplicate of the paper submission, created and submitted on a compact disc. Web the presubmission process can be used to get advice from fda on a range of issues associated with 510 (k), pma, ide,. Web clinical data for premarket submissions. Web how to use the electronic submission template and. Web official fda applications and submissions forms. Electronic regulatory submission and review. You may not even know where to. Web electronic submission template for medical device 510(k) submissions guidance for industry and food and drug administration. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Web official fda applications and submissions forms. Web how to use the electronic submission template and resource (estar) pdf template. Web the estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical. You may not even know where to. Web clinical data for premarket submissions. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. Electronic regulatory submission and review. Web the estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Web pre submission is. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Web the presubmission process can be used to get advice from fda on a range of issues associated with 510 (k), pma, ide,. To understand the various types of requests for fda feedback that are tracked as q. Web pre submission is a way for the. Electronic regulatory submission and review. You may not even know where to. Web electronic submission template for medical device 510(k) submissions guidance for industry and food and drug administration. Web clinical data for premarket submissions. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. Web official fda applications and submissions forms. Web an electronic copy (ecopy) is defined as an exact duplicate of the paper submission, created and submitted on a compact disc. Web the estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical. Web electronic submission template for medical device 510(k) submissions guidance for industry and food and drug administration. The center for devices and radiological health (cdrh) accepts and encourages the. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Web the presubmission process can be used to get advice from fda on a range of issues associated with 510 (k), pma, ide,. Web pre submission is a way for the industry to get the opinion from the fda before the submission of a premarket. Electronic regulatory submission and review. You may not even know where to. Web clinical data for premarket submissions. Web regulatory resources template documents these template documents are meant to serve as a guide for preparation of. Web how to use the electronic submission template and resource (estar) pdf template. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. To understand the various types of requests for fda feedback that are tracked as q. You may not even know where to. Web official fda applications and submissions forms. Web electronic submission template for medical device 510 (k) submissions guidance for industry and. Electronic regulatory submission and review. Web electronic submission template for medical device 510(k) submissions guidance for industry and food and drug administration. Web an electronic copy (ecopy) is defined as an exact duplicate of the paper submission, created and submitted on a compact disc. Without it you could spend a lot of money on studies that the fda won’t consider for clearance. The center for devices and radiological health (cdrh) accepts and encourages the. To understand the various types of requests for fda feedback that are tracked as q. Web the presubmission process can be used to get advice from fda on a range of issues associated with 510 (k), pma, ide,. Web clinical data for premarket submissions. Web how to use the electronic submission template and resource (estar) pdf template.US FDA Prior Notice Form_First Choice Consulting Services
A Quick & Easy Guide to FDA PreSubmissions
FDA 510(k) submission redacted
Sample FDA Prior Notices FDA Prior Notice Submission Service for
FDA 510(k) submission redacted
FDA 510(k) submission redacted
US FDA Prior Notice Form_First Choice Consulting Services
PPT Michael A. Swit, Esq. Vice President PowerPoint Presentation
FDA HFE Submission Report Checklist by UserWise Issuu
510k Cover Letter Template • Invitation Template Ideas
Web The Estar Is An Interactive Pdf Form That Guides Applicants Through The Process Of Preparing A Comprehensive Medical.
Web Regulatory Resources Template Documents These Template Documents Are Meant To Serve As A Guide For Preparation Of.
Web Pre Submission Is A Way For The Industry To Get The Opinion From The Fda Before The Submission Of A Premarket.
Related Post: