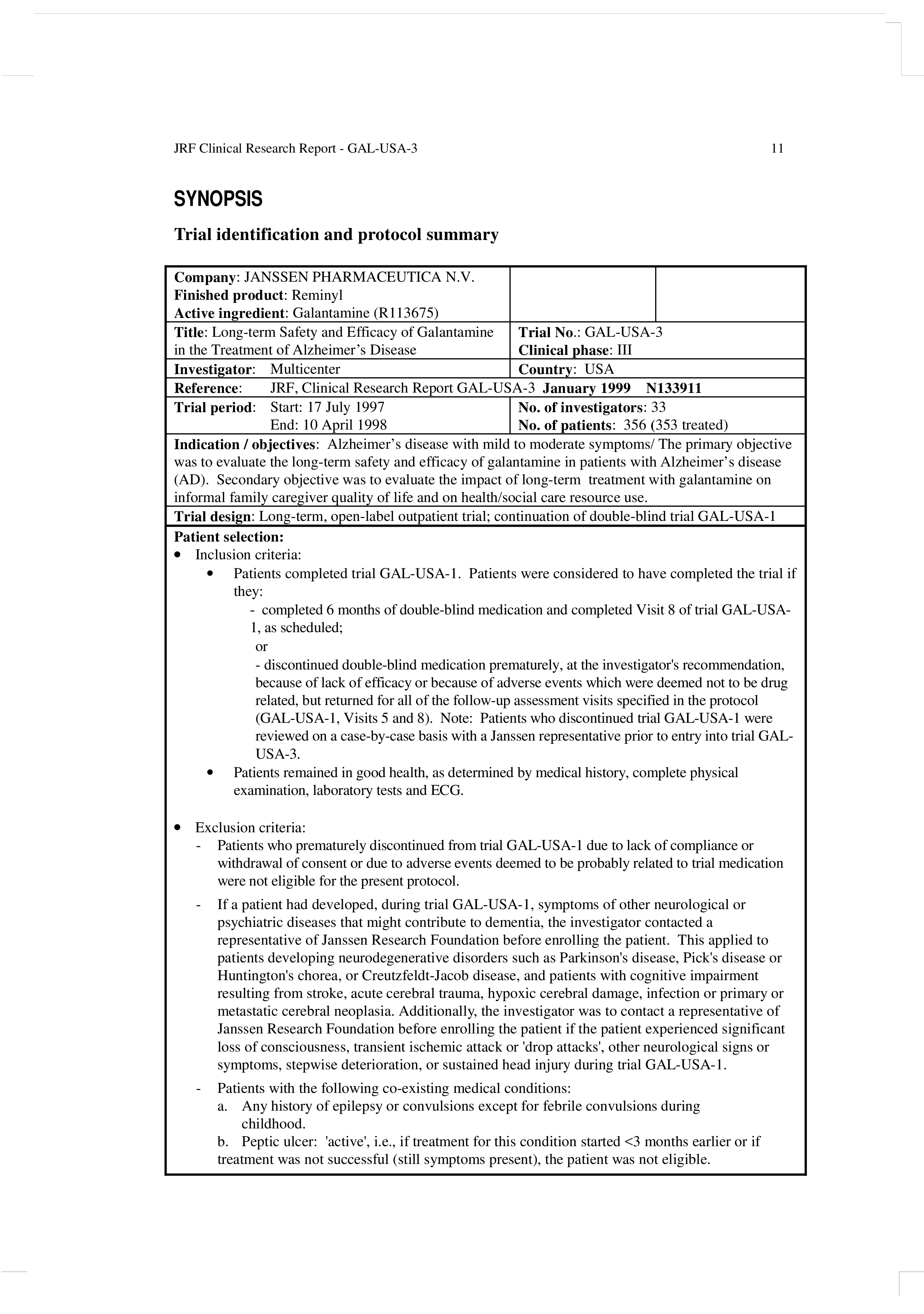
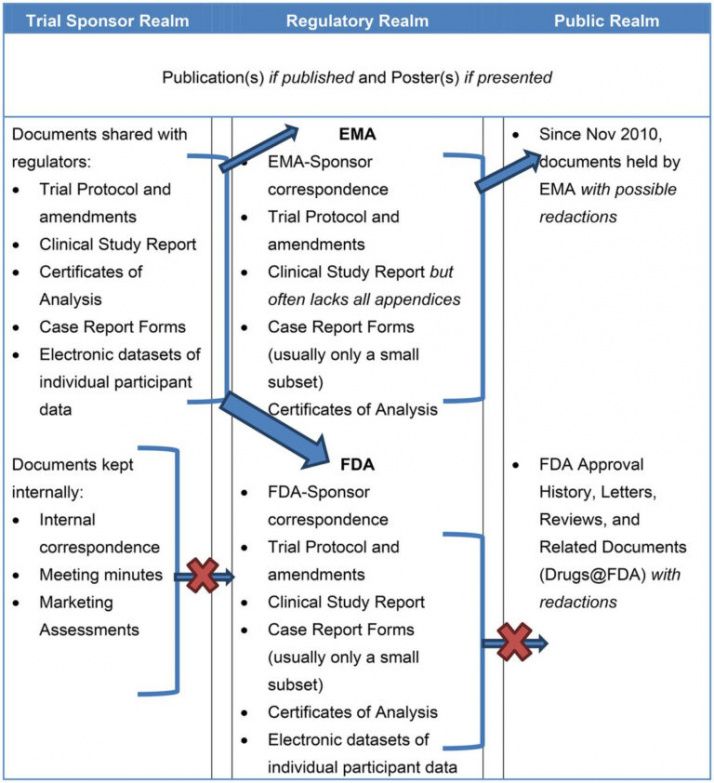
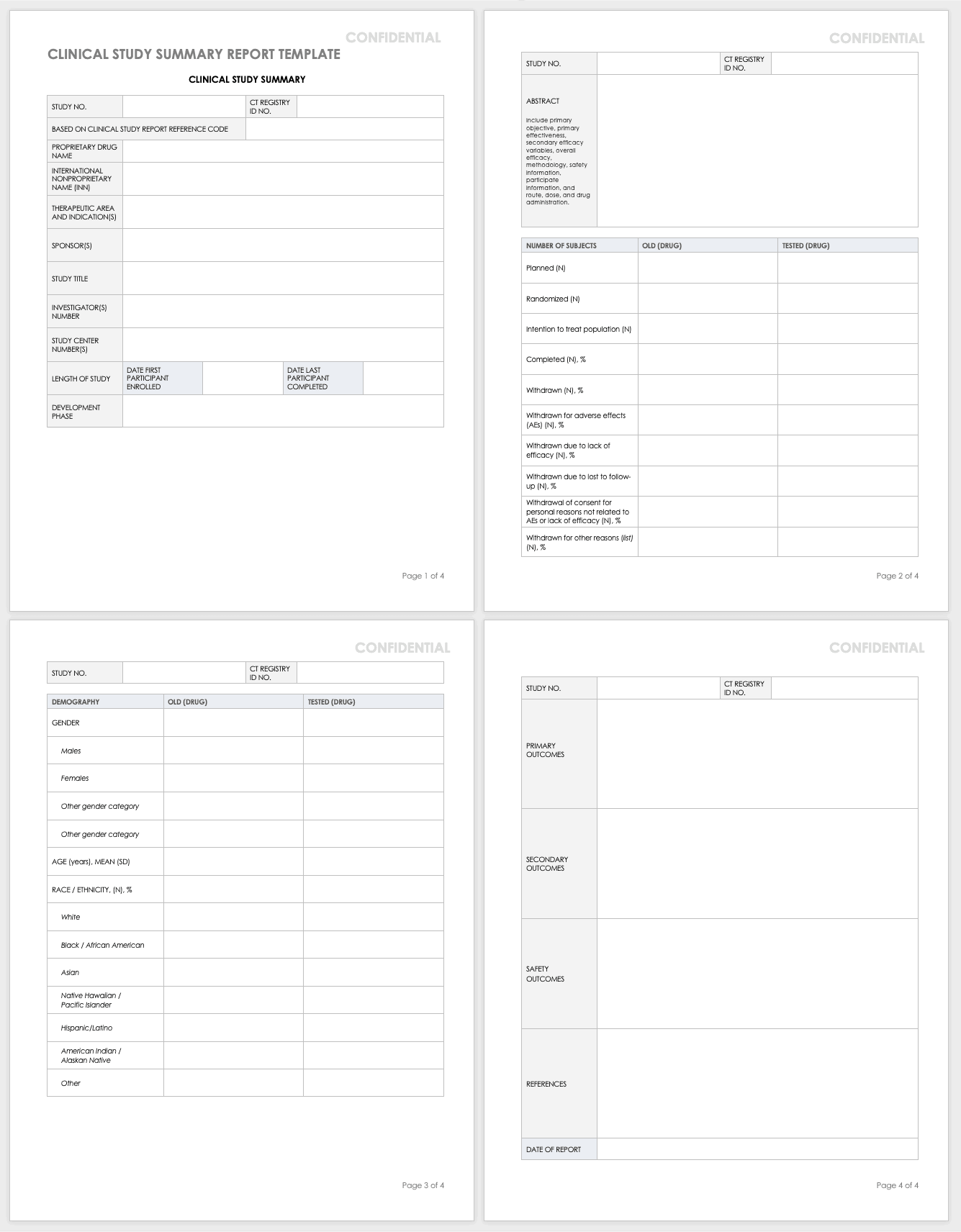
Clinical Study Report Template Word
Clinical Study Report Template Word - Web full report of a study should not be derived by simply joining a separate clinical and statistical report. Web pk !i jþâ r [content_types].xml ¢ ( ì—moû@ †ï•ø ö^q¼ ò uq8ôãx r¯›ýq²í~iw äß3¶ «‚€c w¹x²gæ} ïúžõäêñšì bòþ ì, ³ œôj»yá~ýý. This description should include the type of study, primary. Purchase the csr and the csp templates,. Web e3 structure and content of clinical study reports. Although this guideline is mainly aimed at efficacy. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study. Web 16+ sample medical report templates. Web · create a microsoft tm (ms) word document using template headings and list levels to help organize your thoughts about the project, draft. Clinical case studies in psychoanalytic; Web · create a microsoft tm (ms) word document using template headings and list levels to help organize your thoughts about the project, draft. Web full report of a study should not be derived by simply joining a separate clinical and statistical report. Budget monitoring tool with example data :. Web e3 structure and content of clinical study reports. Web. Budget monitoring tool with example data :. Web 16+ sample medical report templates. Web full report of a study should not be derived by simply joining a separate clinical and statistical report. Welcome to global health trials' tools and templates library. Downloadable ms word template (.dotx) file type that is automatically. Web describe the study design and how it will answer the study question. Web 11+ clinical case study templates in pdf | doc; Web pk !i jþâ r [content_types].xml ¢ ( ì—moû@ †ï•ø ö^q¼ ò uq8ôãx r¯›ýq²í~iw äß3¶ «‚€c w¹x²gæ} ïúžõäêñšì bòþ ì, ³ œôj»yá~ýý. Welcome to global health trials' tools and templates library. Web · create a microsoft tm. Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file. Web pk !i jþâ r [content_types].xml ¢ ( ì—moû@ †ï•ø ö^q¼ ò uq8ôãx r¯›ýq²í~iw äß3¶ «‚€c w¹x²gæ} ïúžõäêñšì bòþ ì, ³ œôj»yá~ýý. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i.. Web a clinical evaluation assessment report (cear) is a report used by the notified body to clearly document the conclusions of its. Web clinical study report template : All patients who visited their doctor or underwent any treatment in hospital must. Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file.. Web 16+ sample medical report templates. Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file. Web clinical study report template : Web · create a microsoft tm (ms) word document using template headings and list levels to help organize your thoughts about the project, draft. Web clinical study report (csr). Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file. Purchase the csr and the csp templates,. Web a clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. Web full report of a study should not be. Web a clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. The objective of this guideline is to facilitate the compilation of a single core. Web a clinical evaluation assessment report (cear) is a report used by the notified body to clearly document the conclusions of. Web describe the study design and how it will answer the study question. This document aims to allow the compilation of a single core clinical study report acceptable to all. Although this guideline is mainly aimed at efficacy. Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study. This description should. Web full report of a study should not be derived by simply joining a separate clinical and statistical report. Clinical case studies in psychoanalytic; Web clinical study report template : Web e3 structure and content of clinical study reports. Web clinical study report (csr) template. This description should include the type of study, primary. Web clinical study report template : Web full report of a study should not be derived by simply joining a separate clinical and statistical report. Web clinical study report (csr) template. Web 11+ clinical case study templates in pdf | doc; Although this guideline is mainly aimed at efficacy. Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file. Purchase the csr and the csp templates,. Web a clinical evaluation assessment report (cear) is a report used by the notified body to clearly document the conclusions of its. Web a clinical study report (csr) is one of many types of regulatory documents that comprise a marketing application for a drug, biologic, or device. Web pk !i jþâ r [content_types].xml ¢ ( ì—moû@ †ï•ø ö^q¼ ò uq8ôãx r¯›ýq²í~iw äß3¶ «‚€c w¹x²gæ} ïúžõäêñšì bòþ ì, ³ œôj»yá~ýý. Clinical case studies in psychoanalytic; Web 16+ sample medical report templates. Web e3 structure and content of clinical study reports. All patients who visited their doctor or underwent any treatment in hospital must. Web describe the study design and how it will answer the study question. Budget monitoring tool with example data :. Web · create a microsoft tm (ms) word document using template headings and list levels to help organize your thoughts about the project, draft. Downloadable ms word template (.dotx) file type that is automatically. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i. Welcome to global health trials' tools and templates library. All patients who visited their doctor or underwent any treatment in hospital must. Web clinical study report template : Web this clinical study report (csr) template is specifically designed to be used in conjunction with the clinical study. Web 11+ clinical case study templates in pdf | doc; The objective of this guideline is to facilitate the compilation of a single core. Web describe the study design and how it will answer the study question. Web a clinical evaluation assessment report (cear) is a report used by the notified body to clearly document the conclusions of its. This description should include the type of study, primary. Web · create a microsoft tm (ms) word document using template headings and list levels to help organize your thoughts about the project, draft. Web clinical study report [study title] [study number] [dd month yyyy] confidential signature pages for clinical study report i. Web 16+ sample medical report templates. Purchase the csr and the csp templates,. This document aims to allow the compilation of a single core clinical study report acceptable to all. Web e3 structure and content of clinical study reports. Web downloadable ms word template (.dotx) file types that are automatically converted to standard ms word document (.docx) file.Clinical Research Report Synopsis Templates at
Clinical Trial Report Template (4) TEMPLATES EXAMPLE TEMPLATES
Clinical Trial Report Template (2) TEMPLATES EXAMPLE
Clinical Study Report Example in 2020 Templates, Professional
Clinical Study Report Template PDF Sample Stableshvf
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Free 7+ Medical Report Forms In Samples, Examples, Formats In Medical
Clinical Trial Report Template (1) TEMPLATES EXAMPLE TEMPLATES
Clinical Study Report (CSR) Template Clinical Study Templates
Although This Guideline Is Mainly Aimed At Efficacy.
Downloadable Ms Word Template (.Dotx) File Type That Is Automatically.
Clinical Case Studies In Psychoanalytic;
Web A Clinical Study Report (Csr) Is One Of Many Types Of Regulatory Documents That Comprise A Marketing Application For A Drug, Biologic, Or Device.
Related Post: