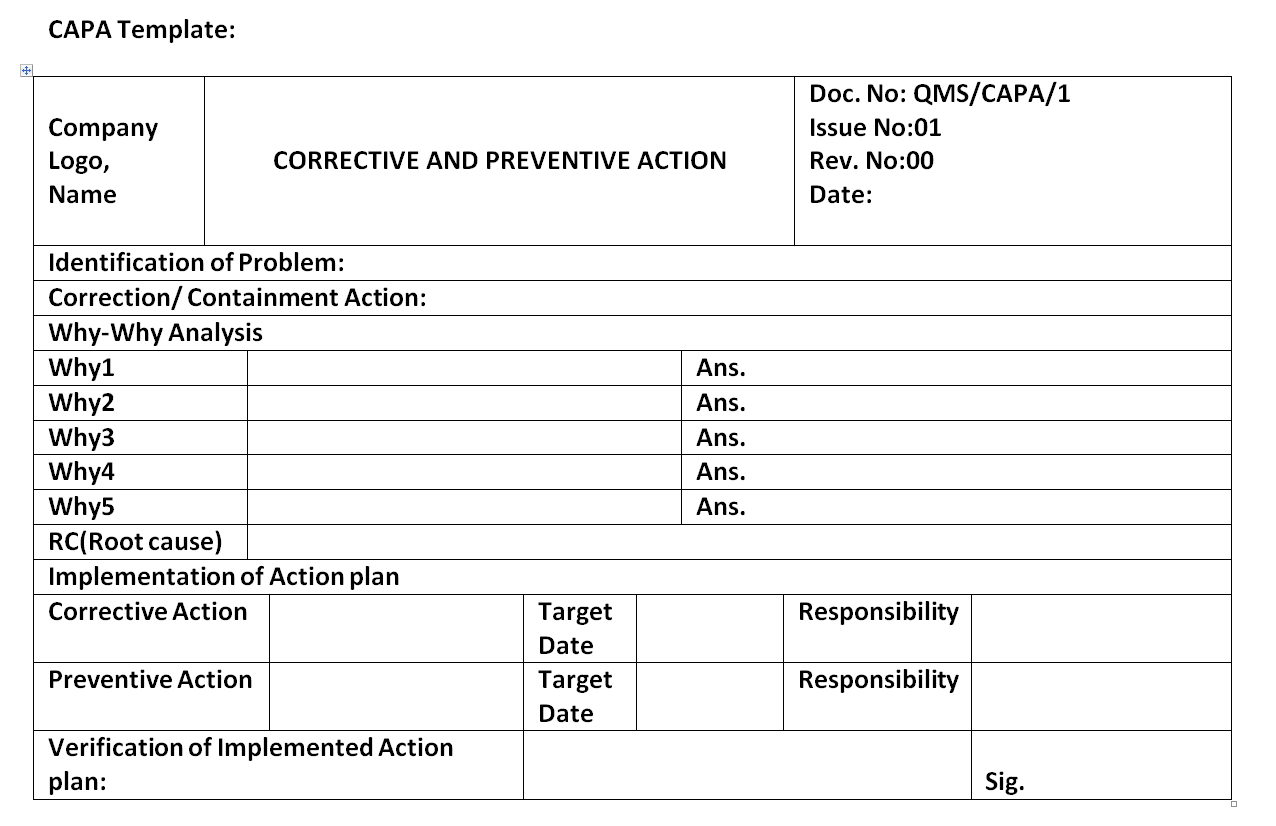
Capa Template Fda
Capa Template Fda - Web corrective action and preventive action plan date effective: Capa is a concept within good manufacturing practices (gmp). (corrective and preventive actions) structured approach to the investigation process should be used. Web capa refers to corrective and preventative actions. The guide to capa & root. Application form is downloaded from www.fda.gov.ph. Added use of qmis form. Get the template who needs capa? Web what is capa per ich q10? Food and drug administration (fda) the fda’s quality system regulations, 21 cfr 820.100. Determine if the firm manufactures or imports a tracked device. 12 april 2016 form no. Food and drug administration (fda) the fda’s quality system regulations, 21 cfr 820.100. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. The integrated application form in xls or xlsx format is. Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from. The integrated application form in xls or xlsx format is. 12 april 2016 form no. Web corrective and preventive actions (capa) form template. Food and drug administration (fda) the fda’s quality system regulations, 21 cfr 820.100. Web medical device tracking. Web capa fda is a quality management strategy used in the manufacturing and production industries to meet the intent of the fda 21 cfr 820.100. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. The integrated application form in xls or xlsx format is.. Added ora capa procedure to reference section. Application form is downloaded from www.fda.gov.ph. Web what is capa per ich q10? (corrective and preventive actions) structured approach to the investigation process should be used. Web it’s stressful to receive an observation or warning letter from the food and drug administration for corrective and. 1) creating and submitting a request; Web complete capa format in excel download in just several minutes by simply following the guidelines listed below: Web capa refers to corrective and preventative actions. Web corrective action and preventive action plan date effective: 12 april 2016 form no. Web it’s stressful to receive an observation or warning letter from the food and drug administration for corrective and. Determine if the firm manufactures or imports a tracked device. Web corrective and preventive actions (capa) form template. 1) creating and submitting a request; Web capa procedures • your firm failed to establish, maintain, and implement a corrective and preventive action. Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from. 12 april 2016 form no. Web capa refers to corrective and preventative actions. Determine if the firm manufactures or imports a tracked device. Web what is capa per ich q10? Web corrective action and preventive action plan date effective: Added use of qmis form. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. Food and drug administration (fda) the fda’s quality system regulations, 21 cfr 820.100. Web a corrective and preventive action (capa) system is a roadmap of. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. Web medical device tracking. Application form is downloaded from www.fda.gov.ph. Get the template who needs capa? 12 april 2016 form no. Added use of qmis form. Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect. Web get started with this customizable capa form template. 12 april 2016 form no. 1) creating and submitting a request; 12 april 2016 form no. The guide to capa & root. Web medical device tracking. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. Web complete capa format in excel download in just several minutes by simply following the guidelines listed below: Web capa refers to corrective and preventative actions. (corrective and preventive actions) structured approach to the investigation process should be used. Web capa fda is a quality management strategy used in the manufacturing and production industries to meet the intent of the fda 21 cfr 820.100. Get the template who needs capa? 1) creating and submitting a request; Food and drug administration (fda) the fda’s quality system regulations, 21 cfr 820.100. Application form is downloaded from www.fda.gov.ph. Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect. The integrated application form in xls or xlsx format is. Web as noted throughout this guide, capa is an important process for your medical device company. Added ora capa procedure to reference section. Capa is a concept within good manufacturing practices (gmp). Web capa procedures • your firm failed to establish, maintain, and implement a corrective and preventive action procedure, as required by 820.100(a). Web corrective and preventive action plan (capa) • a system for resolving quality issues • resolve/correct problem and keep it from. Added use of qmis form. Our free capa form template has all the required fields and is a quick, readymade solution. Web it discusses capa within iso 9001 and within the regulation fda 21 cfr 820 and outlines how and what data. Web capa refers to corrective and preventative actions. The guide to capa & root. Web capa procedures • your firm failed to establish, maintain, and implement a corrective and preventive action procedure, as required by 820.100(a). The integrated application form in xls or xlsx format is. If you’ve ever had a workplace. Web corrective action and preventive action plan date effective: Web a corrective and preventive action (capa) system is a roadmap of processes regulators expect. Web as noted throughout this guide, capa is an important process for your medical device company. Web complete capa format in excel download in just several minutes by simply following the guidelines listed below: Web it’s stressful to receive an observation or warning letter from the food and drug administration for corrective and. Capa is a concept within good manufacturing practices (gmp). Determine if the firm manufactures or imports a tracked device. 1) creating and submitting a request; Get the template who needs capa?LOGO
Corrective and Preventive Action Format CAPA with Example Download
Corrective and preventive action plan CAPA report form
What FDA Expects to See as Part of Your CAPA Process Free Download
Customs & International Trade Law Expert483 Inspection Observation
CAPA form Corrective action and preventive action
LOGO
SOP For Corrective Action and Preventive Actions Pharmaceutical
Medical Audit & CAPA Template brochure
CAPA Solutions Document Control CAPA Management Software
Added Use Of Qmis Form.
Added Ora Capa Procedure To Reference Section.
(Corrective And Preventive Actions) Structured Approach To The Investigation Process Should Be Used.
Web What Is Capa Per Ich Q10?
Related Post: